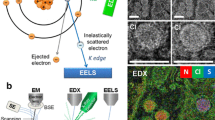
Abstract
The high sensitivity of electron energy-loss spectroscopy (EELS) for detecting light elements at the nanoscale makes it a valuable technique for application to biological systems. In particular, EELS provides quantitative information about elemental distributions within subcellular compartments, specif c atoms bound to individual macromolecular assemblies, and the composition of bionanoparticles. EELS data can be acquired either in the f xed beam energy-filtered transmission electron microscope (EFTEM) or in the scanning transmission electron microscope, and recent progress in the development of both approaches has greatly expanded the range of applications for EELS analysis. Near single atom sensitivity is now achievable for certain elements bound to isolated macromolecules, and it becomes possible to obtain three-dimensional compositional distributions from sectioned cells through EFTEM tomography.











Similar content being viewed by others
References
C. Colliex, N. Brun, A. Gloter, D. Imhoff, M. Kociak, K. March, C. Mory, O. Stéphan, M. Tencé, M. Walls, Philos. Trans. R. Soc. London, Ser. A 367, 3845 (2009).
M. Varela, S.D. Findlay, A.R. Lupini, H.M. Christen, A.Y. Borisevich, N. Dellby, O.L. Krivanek, P.D. Nellist, M.P. Oxley, L.J. Allen, S.J. Pennycook, Phys. Rev. Lett. 92, 95502 (2004).
D.A. Muller, Nat. Mater. 8, 263 (2009).
M.S. Isaacson, in Principles and Techniques of Electron Microscopy, M.A. Hyatt, Ed. ( Van Nostrand-Reinhold, New York, 1977), p. 1.
H. Shuman, A.P. Somlyo, Ultramicroscopy 21, 23 (1987).
M. Isaacson, D. Johnson, Ultramicroscopy 1, 33 (1975).
S. Sun, S. Shi, J.A. Hunt, R.D. Leapman, J. Microsc. 177, 18 (1995).
R.D. Leapman, C.E. Fiori, C.R. Swyt, J. Microsc. 133, 239 (1984).
F.P. Ottensmeyer, J. Ultrastruct. Res. 88, 121 (1984).
D.P. Bazett-Jones, B. Leblanc, M. Herfort, T. Moss, Science 264, 1134 (1994).
D.P. Bazett-Jones, M.J. Hendzel, M.J. Kruhlak, Micron 30, 151 (1999).
R.D. Leapman, N.W. Rizzo, Ultramicroscopy 78, 251 (1999).
C. Colliex, C. Mory, Biol. Cell 80, 175 (1994).
R.D. Leapman, M. Jarnik, A.C. Steven, J. Struct. Biol. 120, 168 (1997).
R.D. Leapman, R.L. Ornberg, Ultramicroscopy 24, 251 (1988).
R.D. Leapman, J.A. Hunt, R.A. Buchanan, S.B. Andrews, Ultramicroscopy 49, 225 (1993).
R.D. Leapman, J. Microsc. 210, 5 (2003).
H. Shuman, Ultramicroscopy 6, 163 (1981).
O.L. Krivanek, C.C. Ahn, R.B. Keeney, Ultramicroscopy 22, 103 (1987).
M.M. van Schooneveld, A. Gloter, O. Stephan, L.F. Zagonel, R. Koole, A. Meijerink, W.J.M. Mulder, F.M.F. de Groot, Nat. Nanotechnol. 5, 538 (2010).
A.A. Sousa, M.A. Aronova, H. Wu, H. Sarin, G.L. Griffiths, R.D. Leapman, Nanomedicine 4, 391 (2009).
R.D. Leapman, S. Sun, Ultramicroscopy 59, 71 (1995).
M.A. Aronova, A.A. Sousa, R.D. Leapman, Micron 42, 252 (2011).
O.L. Krivanek, A.J. Gubbens, N. Dellby, C.E. Meyer, Microsc. Microanal. Microstruct. 3, 187 (1992).
C. Jeanguillaume, C. Colliex, Ultramicroscopy 28, 252 (1989).
J.A. Hunt, D.B. Williams, Ultramicroscopy 38, 47 (1991).
G. Goping, H.B. Pollard, M. Srivastava, R.D. Leapman, Microsc. Res. Tech. 61, 448 (2003).
S. Yakovlev, M. Misra, S. Shi, E. Firlar, M. Libera, Ultramicroscopy 110, 866 (2010).
A. Sousa, A. Aitouchen, M. Libera, Ultramicroscopy 106, 130 (2006).
J. Hongpaisan, N.B. Pivovarova, S.L. Colgrove, R.D. Leapman, D.D. Friel, S.B. Andrews, J. Gen. Physiol. 118, 101 (2001).
M.A. Aronova, Y.C. Kim, N.B. Pivovarova, S.B. Andrews, R.D. Leapman, Ultramicroscopy 109, 201 (2009).
M. Fukunaga, T.-Q. Lia, P. van Gelderen, J.A. de Zwarta, K. Shmuelia, B. Yaoa, J. Lee, D. Maric, M.A. Aronova, G. Zhang, R.D. Leapman, J.F. Schenck, H. Merkle, J.H. Duyn, Proc. Natl. Acad. Sci. U.S.A. 107, 3834 (2010).
J. Xie, F. Zhang, M. Aronova, L. Zhu, X. Lin, Q. Quan, G. Liu, G. Zhang, K.Y. Choi, K. Kim, X. Sun, S. Lee, S. Sun, R. Leapman, X. Chen, ACS Nano 5, 3043 (2011).
P.A. Midgley, M. Weyland, Ultramicroscopy 96, 413 (2003).
R.D. Leapman, E. Kocsis, G. Zhang, T.L. Talbot, P. Laquerriere, Ultramicroscopy 100, 115 (2004).
T. Boudier, J.P. Lechaire, G. Frébourg, C. Messaoudi, C. Mory, C. Colliex, F. Gaill, S. Marco, J. Struct. Biol. 151, 151 (2005).
A.J. Koster, R. Grimm, D. Typke, R. Hegerl, A. Stoschek, J. Walz, W. Baumeister, J. Struct. Biol. 120, 276 (1997).
M.A. Aronova, Y.C. Kim, R.H. Harmon, A.A. Sousa, G. Zhang, R.D. Leapman, J. Struct. Biol. 160, 35 (2007).
J.S. Kremer, D.N. Mastronarde, J.R. McIntosh, J. Struct. Biol. 116, 168 (1997).
M.A. Aronova, Y.C. Kim, G. Zhang, R.D. Leapman, Ultramicroscopy 107, 232 (2007).
M.A. Aronova, A.A. Sousa, G. Zhang, R.D. Leapman, J. Microsc. 239, 223 (2010).
C. Mory, C. Colliex, Ultramicroscopy 28, 339 (1989).
R.D. Leapman, in Transmission Electron Energy Loss Spectroscopy in Materials Science and the EELS Atlas, 2nd Edition, C. Ahn, Ed. (Wiley-VCH, Berlin, 2004), C.3, p. 49.
O.L. Krivanek, M.F. Chisholm, V. Nicolosi, T.J. Pennycook, G.J. Corbin, N. Dellby, M.F. Murfitt, C.S. Own, Z.S. Szilagyi, M.P. Oxley, S.T. Pantelides, S.J. Pennycook, Nature 464, 571 (2010).
R.F. Egerton, F. Wang, P.A. Crozier, Microsc. Microanal. 12, 65 (2006).
P. Schlossmacher, D.O. Klenov, B. Freitag, H.S. Harrach, Microsc. Today 18, 14 (2010).
A.V. Somlyo, R. Broderick, H. Shuman, E.L. Buhle Jr., A.P. Somlyo, Proc. Natl. Acad. Sci. U.S.A. 85, 6222 (1988).
R.D. Powell, J.F. Hainfeld, Microsc. Res. Tech. 42, 2 (1998)
J.M. Robinson, T. Takizawa, J. Microsc. 235, 259 (2009).
Acknowledgements
This work was supported by the Intramural Research Programs of the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health. The authors thank Dr. Alioscka Sousa (NIBIB, NIH) and Dr. S. Brian Andrews (NINDS, NIH) for valuable discussions and Dr. Guofeng Zhang for his help with specimen preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aronova, M.A., Leapman, R.D. Development of electron energy-loss spectroscopy in the biological sciences. MRS Bulletin 37, 53–62 (2012). https://doi.org/10.1557/mrs.2011.329
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrs.2011.329