Abstract
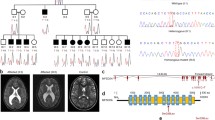
The major facilitator superfamily domain-containing protein 2A (MFSD2A) is a constituent of the blood-brain barrier and functions to transport lysophosphatidylcholines (LPCs) into the central nervous system. LPCs such as that derived from docosahexanoic acid (DHA) are indispensable to neurogenesis and maintenance of neurons, yet cannot be synthesized within the brain and are dependent on MFSD2A for brain uptake. Recent studies have implicated MFSD2A mutations in lethal and non-lethal microcephaly syndromes, with the severity correlating to the residual activity of the transporter. We describe two siblings with shared parental ancestry, in whom we identified a homozygous missense mutation (c.1205C > A; p.Pro402His) in MFSD2A. Both affected individuals had microcephaly, hypotonia, appendicular spasticity, dystonia, strabismus, and global developmental delay. Neuroimaging revealed paucity of white matter with enlarged lateral ventricles. Plasma lysophosphatidylcholine (LPC) levels were elevated, reflecting reduced brain transport. Cell-based studies of the p.Pro402His mutant protein indicated complete loss of activity of the transporter despite the non-lethal, attenuated phenotype. The aggregate data of MFSD2A-associated genotypes and phenotypes suggest that additional factors, such as nutritional supplementation or modifying genetic factors, may modulate the severity of disease and call for consideration of treatment options for affected individuals.




Similar content being viewed by others
References
Zhao Z, Zlokovic BV (2014) Blood-brain barrier: a dual life of MFSD2A? Neuron 82:728–730
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201
Nałęcz KA (2016) Solute carriers in the blood-brain barier: safety in abundance. Neurochem Res 42:795–809
Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL (2014) Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509:503–506
Guemez-Gamboa, A., Nguyen, L.N., Yang, H., Zaki, M.S., Kara, M., Ben-Omran, T., Akizu, N., Rosti, R.O., Rosti, B., Scott, E., et al. (2015). Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet 47, 809–813
Kawakita E, Hashimoto M, Shido O (2006) Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience 139:991–997
He C, Qu X, Cui L, Wang J, Kang JX (2009) Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci U S A 106:11370–11375
Coti Bertrand P, O'Kusky JR, Innis SM (2006) Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr 136:1570–1575
Gharami K, Das M, Das S (2015) Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem Int 89:51–62
Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C (2014) Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509:507–511
Quek DQ, Nguyen LN, Fan H, Silver DL (2016) Structural insights into the transport mechanism of the human sodium-dependent lysophosphatidylcholine transporter MFSD2A. J Biol Chem 291:9383–9394
Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C (2017) Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 94:581–594.e585
Alakbarzade, V., Hameed, A., Quek, D.Q., Chioza, B.A., Baple, E.L., Cazenave-Gassiot, A., Nguyen, L.N., Wenk, M.R., Ahmad, A.Q., Sreekantan-Nair, A., et al. (2015). A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet 47, 814–817
Berger JH, Charron MJ, Silver DL (2012) Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS One 7:e50629
Krivov GG, Shapovalov MV, Dunbrack RL (2009) Improved prediction of protein side-chain conformations with SCWRL4. Proteins 77:778–795
Sali A, Blundell TL (1993) Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815
Shen MY, Sali A (2006) Statistical potential for assessment and prediction of protein structures. Protein Sci 15:2507–2524
Mysinger MM, Shoichet BK (2010) Rapid context-dependent ligand desolvation in molecular docking. J Chem Inf Model 50:1561–1573
Vanderver A, Prust M, Tonduti D, Mochel F, Hussey HM, Helman G, Garbern J, Eichler F, Labauge P, Aubourg P et al (2015) Case definition and classification of leukodystrophies and leukoencephalopathies. Mol Genet Metab 114:494–500
Reddy VS, Shlykov MA, Castillo R, Sun EI, Saier MH (2012) The major facilitator superfamily (MFS) revisited. FEBS J 279:2022–2035
Seidner G, Alvarez MG, Yeh JI, O'Driscoll KR, Klepper J, Stump TS, Wang D, Spinner NB, Birnbaum MJ, De Vivo DC (1998) GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat Genet 18:188–191
Klepper J, Voit T (2002) Facilitated glucose transporter protein type 1 (GLUT1) deficiency syndrome: impaired glucose transport into brain-- a review. Eur J Pediatr 161:295–304
Siintola E, Topcu M, Aula N, Lohi H, Minassian BA, Paterson AD, Liu XQ, Wilson C, Lahtinen U, Anttonen AK et al (2007) The novel neuronal ceroid lipofuscinosis gene MFSD8 encodes a putative lysosomal transporter. Am J Hum Genet 81:136–146
Meyer E, Ricketts C, Morgan NV, Morris MR, Pasha S, Tee LJ, Rahman F, Bazin A, Bessières B, Déchelotte P et al (2010) Mutations in FLVCR2 are associated with proliferative vasculopathy and hydranencephaly-hydrocephaly syndrome (fowler syndrome). Am J Hum Genet 86:471–478
Salomons GS, van Dooren SJ, Verhoeven NM, Cecil KM, Ball WS, Degrauw TJ, Jakobs C (2001) X-linked creatine-transporter gene (SLC6A8) defect: a new creatine-deficiency syndrome. Am J Hum Genet 68:1497–1500
van de Kamp JM, Betsalel OT, Mercimek-Mahmutoglu S, Abulhoul L, Grünewald S, Anselm I, Azzouz H, Bratkovic D, de Brouwer A, Hamel B et al (2013) Phenotype and genotype in 101 males with X-linked creatine transporter deficiency. J Med Genet 50:463–472
Dunbar M, Jaggumantri S, Sargent M, Stockler-Ipsiroglu S, van Karnebeek CD (2014) Treatment of X-linked creatine transporter (SLC6A8) deficiency: systematic review of the literature and three new cases. Mol Genet Metab 112:259–274
Srour M, Hamdan FF, Gan-Or Z, Labuda D, Nassif C, Oskoui M, Gana-Weisz M, Orr-Urtreger A, Rouleau GA, Michaud JL (2015) A homozygous mutation in SLC1A4 in siblings with severe intellectual disability and microcephaly. Clin Genet 88:e1–e4
Damseh N, Simonin A, Jalas C, Picoraro JA, Shaag A, Cho MT, Yaacov B, Neidich J, Al-Ashhab M, Juusola J et al (2015) Mutations in SLC1A4, encoding the brain serine transporter, are associated with developmental delay, microcephaly and hypomyelination. J Med Genet 52:541–547
Heimer G, Marek-Yagel D, Eyal E, Barel O, Oz Levi D, Hoffmann C, Ruzzo EK, Ganelin-Cohen E, Lancet D, Pras E et al (2015) SLC1A4 mutations cause a novel disorder of intellectual disability, progressive microcephaly, spasticity and thin corpus callosum. Clin Genet 88:327–335
El-Hattab AW (2016) Serine biosynthesis and transport defects. Mol Genet Metab 118:153–159
Acknowledgements
The authors wish to thank the family for their participation in this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The work was supported in part by National Research Foundation grants, Singapore NRF2016NRF-NRFI001-15 (to D.L.S.), NRFI2015-05 (to M.R.W.); by the Biomedical Research Council of A*STAR (to H. F.); and by a BMRC-SERC joint grant (BMRC-SERC 112 148 0006, to M.R.W.) from the Agency for Science, Technology and Research, Singapore.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Harel, T., Quek, D.Q.Y., Wong, B.H. et al. Homozygous mutation in MFSD2A, encoding a lysolipid transporter for docosahexanoic acid, is associated with microcephaly and hypomyelination. Neurogenetics 19, 227–235 (2018). https://doi.org/10.1007/s10048-018-0556-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-018-0556-6