Abstract
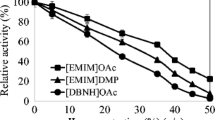
β-Glucosidase (BG) hydrolyzes cellobiose into glucose, and is a vital step in converting ionic liquids (ILs)-pretreated biomass to sustainable biofuels. The inactivation mechanism of BG from Paenibacillus sp. LLZ1 induced by microcrystalline cellulose was explored in various concentrations of ILs, composed of [Emim]+ cation and [DEP]−, [OAc]−, [Br]−, [Cl]−, and [BF4]− anions. The FTIR analysis of inactivated BG indicated that the ILs altered its β-sheet content. Moreover, circular dichroism spectroscopy (CD) suggested that the α-helix content decreased, while the β-sheet content increased with the presence of ILs in general. Interestingly, the secondary structure of BG had almost no change after [Emim]DEP treatment, while ionic liquid [Emim]BF4 treatment caused the irreversible denaturation of BG. Eventually, by adding 0.4 mM of Aerosol OT surfactant, the BG activity was increased by 20.1% in the presence of 25% [Emim]DEP, and the corresponding glucose yield from hydrolysis of cellobiose was increased by 23.9%.









Similar content being viewed by others
References
Ling, Z., Chen, S., Zhang, X., Takabe, K., & Xu, F. (2017). Unraveling variations of crystalline cellulose induced by ionic liquid and their effects on enzymatic hydrolysis. Scientific Reports, 7(1), 1–11.
Wang, Y., Yu, W., & Han, F. (2016). Expression and characterization of a cold-adapted, thermotolerant and denaturant-stable GH5 endoglucanase Celal_2753 that withstands boiling from the psychrophilic bacterium Cellulophaga algicola IC166T. Biotechnology Letters, 38(2), 285–290.
Zhang, Q., Zhao, M., Xu, Q., Ren, H. R., & Yin, J. Z. (2019). Enhanced enzymatic hydrolysis of sorghum stalk by supercritical carbon dioxide and ultrasonic pretreatment. Applied Biochemistry and Biotechnology, 188(1), 101–111.
Liu, L., Li, Z., Hou, W., & Shen, H. (2018). Direct conversion of lignocellulose to levulinic acid catalyzed by ionic liquid. Carbohydrate Polymers, 181, 778–784.
Plechkova, N. V., & Seddon, K. R. (2008). Applications of ionic liquids in the chemical industry. Chemical Society Reviews, 37(1), 123–150.
Hou, Q. D., Ju, M. T., Li, W. Z., Liu, L., Chen, Y., & Yang, Q. (2017). Pretreatment of lignocellulosic biomass with ionic liquids and ionic liquid-based solvent systems. Molecules, 22(490), 1–24.
Olivier-Bourbigou, H., Magna, L., & Morvan, D. (2010). Ionic liquids and catalysis: recent progress from knowledge to applications. Applied Catalysis A-General, 373(1-2), 1–56.
Feng, D., Li, L. Z., Fang, Y., Tan, W., Zhao, G., Zou, H., Xian, M., & Zhang, Y. (2011). Separation of ionic liquid [Mmim][DMP] and glucose from enzymatic hydrolysis mixture of cellulose using alumina column chromatography. Applied Microbiology and Biotechnology, 91(2), 399–405.
Zanphorlin, L. M., de Giuseppe, P. O., Honorato, R. V., Tonoli, C. C., Fattori, J., Crespim, E., de Oliveira, P. S., Ruller, R., & Murakami, M. T. (2016). Oligomerization as a strategy for cold adaptation: structure and dynamics of the GH1 beta-glucosidase from Exiguobacterium antarcticum B7. Scientific Reports, 6, 1–14.
Salgado, J. C. S., Meleiro, L. P., Carli, S., & Ward, R. J. (2018). Glucose tolerant and glucose stimulated β-glucosidases – a review. Bioresource Technology, 267, 704–713.
Dong, W. L., Xue, M. L., Zhang, Y., Xin, F. X., Wei, C., Zhang, W. M., Wu, H., Ma, J. F., & Jiang, M. (2017). Characterization of a beta-glucosidase from Paenibacillus species and its application for succinic acid production from sugarcane bagasse hydrolysate. Bioresource Technology, 241, 309–316.
Ichikawa, S., Ichihara, M., Ito, T., Isozaki, K., Kosugi, A., & Karita, S. (2018). Glucose production from cellulose through biological simultaneous enzyme production and saccharification using recombinant bacteria expressing the β-glucosidase gene. Journal of Bioscience and Bioengineering, 1–5.
Amaike Campen, S., Lynn, J., Sibert, S. J., Srikrishnan, S., Phatale, P., Feldman, T., Guenther, J. M., Hiras, J., Tran, Y. T. A., Singer, S. W., Adams, P. D., Sale, K. L., Simmons, B. A., Baker, S. E., Magnuson, J. K., & Gladden, J. M. (2017). Expression of naturally ionic liquid-tolerant thermophilic cellulases in Aspergillus niger. PloS One, 12(12), 1–15.
Goswami, S., Gupta, N., & Datta, S. (2016). Using the beta-glucosidase catalyzed reaction product glucose to improve the ionic liquid tolerance of beta-glucosidases. Biotechnol Biofuels, 9(1), 72.
Sun, X., Zhu, L., Wang, J., Wang, J., Su, B., Liu, T., Zhang, C., Gao, C., & Shao, Y. (2017). Toxic effects of ionic liquid 1-octyl-3-methylimidazolium tetrafluoroborate on soil enzyme activity and soil microbial community diversity. Ecotoxicology and Environmental Safety, 135, 201–208.
Fernandez-Lorente, G., Cabrera, Z., Godoy, C., Fernandez-Lafuente, R., Palomo, J. M., & Guisan, J. M. (2008). Interfacially activated lipases against hydrophobic supports: effect of the support nature on the biocatalytic properties. Process Biochemistry, 43(10), 1061–1067.
Abuin, E., Lissi, E., & Duarte, R. (2005). Kinetics of N-glutaryl-L-phenylalanine p-nitroanilide hydrolysis catalyzed by α-chymotrypsin in aqueous solutions of dodecyltrimethylammonium bromide. Journal of Colloid & Interface Science, 283(2), 539–543.
Azimi, M., Nafissi-Varcheh, N., Mogharabi, M., Faramarzi, M. A., & Aboofazeli, R. (2016). Study of laccase activity and stability in the presence of ionic and non-ionic surfactants and the bioconversion of indole in laccase-TX-100 system. Journal of Molecular Catalysis B: Enzymatic, 126, 69–75.
Fan, L. L., Xie, P. J., Wang, Y., Huang, Z. S., & Zhou, J. Z. (2018). Biosurfactant-protein interaction: influences of mannosylerythritol lipids-A on beta-glucosidase. Journal of Agricultural and Food Chemistry, 66(1), 238–246.
Hu, D. X., Xiao, L., Li, L. Z., Zhong, C., Ju, X., Yan, L. S., Wu, T. Y., Qing, M., & Hu, Z. Y. (2016). Effects of ionic liquid 1-ethyl-3-methylimidazolium diethylphosphate on cellulase produced by Paenibacillus sp. LLZ1. ACS Sustainable Chemistry & Engineering, 4(9), 4922–4926.
Hu, D. X., Ju, X., Li, L. Z., Hu, C. Y., Yan, L. S., Wu, T. Y., Fu, J. L., & Qin, M. (2016). Improved in situ saccharification of cellulose pretreated by dimethyl sulfoxide/ionic liquid using cellulase from a newly isolated Paenibacillus sp. LLZ1. Bioresource Technology, 201, 8–14.
Bradford, M. M. (1976). A rapid method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248–254.
Grosch, J. H., Loderer, C., Jestel, T., Marion, A., & Antje, C. (2015). Carbonyl reductase of Candida parapsilosis – stability analysis and stabilization strategy. Journal of Molecular Catalysis B: Enzymatic, 112, 45–53.
Xu, D. X., Zhang, J. J., Cao, Y. P., Wang, J., & Xiao, J. S. (2016). Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. Food Science and Technology, 66, 590–597.
Fan, L., Wang, S. J., & Li, K. L. (2015). Function of imidazolium-based ionic liquids in system of enzymatic degradation of cellulose. CIESC Journal, 66(1), 121–125.
de Eugenio, L. I., Méndez-Líter, J. A., Nieto-Domínguez, M., Alonso, L., Gil-Muñoz, J., Barriuso, J., Prieto, A., & Martínez, M. J. (2017). Differential β-glucosidase expression as a function of carbon source availability in Talaromyces amestolkiae: a genomic and proteomic approach. Biotechnology for Biofuels, 10(1).
Behera, K., & Pandey, S. (2009). Interaction between ionic liquid and zwitterionic surfactant: a comparative study of two ionic liquids with different anions. Journal of Colloid and Interface Science, 331(1), 196–205.
Naushad, M., Alothman, Z. A., Khan, A. B., & Ali, M. (2012). Effect of ionic liquid on activity, stability, and structure of enzymes: a review. International Journal of Biological Macromolecules, 51(4), 555–560.
Vidya, P., & Chadha, A. (2009). The role of different anions in ionic liquids on Pseudomonas cepacia lipase catalyzed transesterification and hydrolysis. Journal of Molecular Catalysis B: Enzymatic, 57(1-4), 145–148.
Gruian, C., Vanea, E., Simon, S., & Simon, V. (2012). FTIR and XPS studies of protein adsorption onto functionalized bioactive glass. Biochimica et Biophysica Acta, 1824(1), 873–881.
Yang, Z., Deng, J., & Chen, L. F. (2007). Effect of ionic and non-ionic surfactants on the activity and stability of mushroom tyrosinase. Journal of Molecular Catalysis B: Enzymatic, 47(1-2), 79–85.
Hansted, J. G., Wejse, P. L., Bertelsen, H., & Otzen, D. E. (2011). Effect of protein-surfactant interactions on aggregation of beta-lactoglobulin. Biochimica et Biophysica Acta - Proteins and Proteomics, 1814(5), 713–723.
Li, S., Du, J., Sun, J., Galazka, J. M., Glass, N. L., Cate, J. H., Yang, X., & Zhao, H. (2010). Overcoming glucose repression in mixed sugar fermentation by co-expressing a cellobiose transporter and a beta-glucosidase in Saccharomyces cerevisiae. Molecular Biosystems, 6(11), 2129–2132.
Wang, M., & Lu, X. (2016). Exploring the synergy between cellobiose dehydrogenase from Phanerochaete chrysosporium and cellulase from Trichoderma reesei. Frontiers in Microbiology, 7, 620.
Funding
The authors are grateful for the financial support from the National Natural Science Foundation of China (Grant No. 21676173 and Grant No. 21376156). This study was also supported by the Qing Lan Project of Jiangsu Education Department. The authors are grateful for the financial support from the agricultural infrastructure project of Suzhou Science and Technology Development Plan (SNG2018046).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, H., Zhou, M., Ju, X. et al. Inactivation Mechanism of 1-Ethyl-3-Methylimidazolium-Based Ionic Liquid on β-Glucosidase Produced by Paenibacillus sp. LLZ1 and Enhanced Activity Using a Surfactant. Appl Biochem Biotechnol 190, 826–838 (2020). https://doi.org/10.1007/s12010-019-03131-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03131-w