Abstract
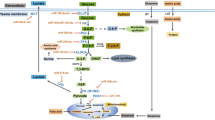
Purines, among most influential molecules, are reported to have essential biological function by regulating various cell types. A large number of studies have led to the discovery of many biological functions of the purine nucleotides such as ATP, ADP, and adenosine, as signaling molecules that engage G protein-coupled or ligand-gated ion channel receptors. The role of purines in the regulation of cellular functions at the gene or protein level has been well documented. With the advances in multiomics, including those from metabolomic and bioinformatic analyses, metabolic reprogramming was identified as a key mechanism involved in the regulation of cellular function under physiological or pathological conditions. Recent studies suggest that purines or purine-derived products contribute to important regulatory functions in many fundamental biological and pathological processes related to metabolic reprogramming. Therefore, this review summarizes the role and potential mechanism of purines in the regulation of metabolic reprogramming. In particular, the molecular mechanisms of extracellular purine- and intracellular purine-mediated metabolic regulation in various cells during disease development are discussed. In summary, our review provides an extensive resource for studying the regulatory role of purines in metabolic reprogramming and sheds light on the utilization of the corresponding peptides or proteins for disease diagnosis and therapy.



Similar content being viewed by others
References
Warburg O (1956) On the origin of cancer cells. Science 123(3191):309–314
Wind F, Negelein E (1927) The metabolism of tumors in the body. J Gen Physiol 8(6):519–530
Ramaiah A (1974) Pasteur effect and phosphofructokinase. Curr Top Cell Regul 8:297–345
Yoshida GJ (2015) Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res 34(1):1–10
Krebs HA (1972) The Pasteur effect and the relations between respiration and fermentation. Essays Biochem 8(1):1
Ward PS, Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21(3):297–308
Libby CJ (2018) The Pro-tumorigenic Effects of Metabolic Alterations in Glioblastoma Including Brain Tumor Initiating Cells. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 1869(2):175–188
Herranz D et al (2015) Metabolic reprogramming induces resistance to anti-NOTCH1 therapies in acute lymphoblastic leukemia. Nat Med 21(10):1182
Rozovski U et al (2016) Metabolism pathways in chronic lymphocytic leukemia. Leuk Lymphoma 57(4):758–765
Boroughs LK, Deberardinis RJ (2015) Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol 17(4):351–359
Aguilar E et al (2016) Metabolic reprogramming and dependencies associated with epithelial cancer stem cells independent of the epithelial-mesenchymal transition program. Stem Cells 34(5):1163
Wettersten HI, Hakimi AA, Morin D, Bianchi C, Johnstone ME, Donohoe DR, Trott JF, Aboud OA, Stirdivant S, Neri B, Wolfert R, Stewart B, Perego R, Hsieh JJ, Weiss RH (2015) Grade-dependent metabolic reprogramming in kidney cancer revealed by combined proteomics and metabolomics analysis. Cancer Res 75(12):2541–2552
Chang HJ et al (2009) GLUT1 gene is a potential hypoxic marker in colorectal cancer patients. BMC Cancer 9(1):241
Dong J, Xiao D, Zhao Z, Ren P, Li C, Hu Y, Shi J, Su H, Wang L, Liu H, Li B, Gao P, Qing G (2017) Epigenetic silencing of microRNA-137 enhances ASCT2 expression and tumor glutamine metabolism. Oncogenesis 6(7):e356
Deberardinis RJ et al (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7(1):11–20
Søreide K, Sund M (2015) Epidemiological-molecular evidence of metabolic reprogramming on proliferation, autophagy and cell signaling in pancreas cancer. Cancer Lett 356(2):281–288
Sinclair LV et al (2013) Corrigendum: control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol 14(5):500–508
Zeng H (2013) T cell exit from quiescence and differentiation into Th2 cells depend on raptor-mTORC1-mediated metabolic reprogramming. Immunity 39(6):1043–1056
Ryall JG, Cliff T, Dalton S, Sartorelli V (2015) Metabolic reprogramming of stem cell epigenetics. Cell Stem Cell 17(6):651–662
Eng CH, Abraham RT (2011) The autophagy conundrum in cancer: influence of tumorigenic metabolic reprogramming. Oncogene 30(47):4687–4696
Subhra NBSP, Biswas K (2015) Metabolic reprogramming of immune cells in cancer progression. Immunity 43(3):435
Kelly B, O'Neill LA (2015) Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res 25(7):771–784
Torigoe M, Iwata S (2017) Metabolic Reprogramming Commits Differentiation of Human CD27(+)IgD(+) B Cells to Plasmablasts or CD27(-)IgD(-) Cells. J Immunol 199(2):425–434
Deng J et al (2016) Homocysteine activates B cells via regulating PKM2-dependent metabolic reprogramming. J Immunol 198(1):170
Al-Khami AA, Rodriguez PC (2016) Metabolic Reprogramming of Myeloid-derived Suppressor Cells (MDSC) in Cancer. OncoImmunology 5(8):e1200771
Keating SE, Zaiatz-Bittencourt V, Loftus RM, Keane C, Brennan K, Finlay DK, Gardiner CM (2016) Metabolic reprogramming supports IFN-γ production by CD56bright NK cells. J Immunol 196(6):2552–2560
Hsu YC, Chen CT, Wei YH (2016) Mitochondrial resetting and metabolic reprogramming in induced pluripotent stem cells and mitochondrial disease modeling. Biochim Biophys Acta 1860(4):686–693
Chen C, Tang Q, Zhang Y, Dai M, Jiang Y, Wang H, Yu M, Jing W, Tian W (2017) Metabolic reprogramming by HIF-1 activation enhances survivability of human adipose-derived stem cells in ischaemic microenvironments. Cell Prolif 50(5):e12363
Oburoglu et al (2014) Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell Stem Cell 15(2):169–184
Liu H, Zhang Y, Wu H, D’Alessandro A, Yegutkin GG, Song A, Sun K, Li J, Cheng NY, Huang A, Edward Wen Y, Weng TT, Luo F, Nemkov T, Sun H, Kellems RE, Karmouty-Quintana H, Hansen KC, Zhao B, Subudhi AW, Jameson-van Houten S, Julian CG, Lovering AT, Eltzschig HK, Blackburn MR, Roach RC, Xia Y (2016) Beneficial role of erythrocyte adenosine A2B receptor-mediated AMP-activated protein kinase activation in high-altitude hypoxia. Circulation 134(5):405–421
Kjaergaard J et al (2018) A 2A adenosine receptor gene deletion or synthetic A 2A antagonist liberate tumor-reactive CD8 + T cells from tumor-induced immunosuppression. J Immunol 201(2):782–791
Carey BW et al (2014) Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518(7539):413–416
Ryall JG, Dell’Orso S, Derfoul A, Juan A, Zare H, Feng X, Clermont D, Koulnis M, Gutierrez-Cruz G, Fulco M, Sartorelli V (2015) The NAD(+)-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell 16(2):171–183
Shyhchang N et al (2013) Lin28 enhances tissue repair by reprogramming cellular metabolism. Cell 155(4):778–792
Michelet X (2018) Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol 19(12):1330–1340
Chang CH, Curtis JD, Maggi LB Jr, Faubert B, Villarino AV, O’Sullivan D, Huang SCC, van der Windt GJW, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL (2013) Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153(6):1239–1251
Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, Nichols AG, Warmoes MO, de Cubas AA, MacIver NJ, Locasale JW, Turka LA, Wells AD, Rathmell JC (2016) Foxp3 and toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat Immunol 17(12):1459–1466
Sica A, Strauss L (2017) Energy metabolism drives myeloid-derived suppressor cell differentiation and functions in pathology. J Leukoc Biol 102(2):325–334
Bettencourt IA, Powell JD (2017) Targeting metabolism as a novel therapeutic approach to autoimmunity, inflammation, and transplantation. J Immunol 198(3):999
Binger KJ, Côrtereal BF (2017) Immunometabolic Regulation of Interleukin-17-Producing T Helper Cells: Uncoupling New Targets for Autoimmunity. Front Immunol 8:311
Huang L, Xu H, Peng G (2018) TLR-mediated metabolic reprogramming in the tumor microenvironment: potential novel strategies for cancer immunotherapy. Cell Mol Immunol 15:428–437
Doherty JR, Cleveland JL (2013) Targeting lactate metabolism for cancer therapeutics. J Clin Invest 123(9):3685–3692
Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, Li L, Boussiotis VA (2015) PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun 6:6692
Noman MZ et al (2014) PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211(5):781–790
Sun K et al (2016) Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat Commun 7:12086
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24(24):509–581
Knowles JR (1980) Enzyme-catalyzed phosphoryl transfer reactions. Annu Rev Biochem 49(1):877–919
Liu H, Xia Y (2015) Beneficial and detrimental role of adenosine signaling in diseases and therapy. J Appl Physiol (1985) 119(10):1173
Mcdonough KA, Rodriguez A (2012) The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol 10(1):27–38
Ferrari D et al (2016) Purinergic signaling during immune cell trafficking. Trends Immunol 37(6):399–411
Antonioli L et al (2008) Regulation of enteric functions by adenosine: pathophysiological and pharmacological implications. Pharmacol Ther 120(3):233–253
Surprenant A, Rassendren F, Kawashima E, North RA, Buell G (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272(5262):735–738
Adinolfi E et al (2017) The P2X7 receptor: a main player in inflammation. Biochem Pharmacol 151:S0006295217307335
Eltzschig HK (2013) Extracellular adenosine signaling in molecular medicine. J Mol Med (Berl) 91(2):141–146
Geldenhuys WJ et al (2017) Exploring adenosine receptor ligands: potential role in the treatment of cardiovascular diseases. Molecules 22(6):917
Haskó G, Csóka B, Németh ZH, Vizi ES, Pacher P (2009) A2B adenosine receptors in immunity and inflammation. Trends Immunol 30(6):263–270
Abbracchio MP, Burnstock G (1994) Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther 64(3):445–475
Jarvis MF, Khakh BS (2009) ATP-gated P2X cation-channels. Neuropharmacology 56(1):208–215
Idzko M, Ferrari D, Eltzschig HK (2014) Nucleotide signalling during inflammation. Nature 509(7500):310–317
Cekic C, Linden J (2016) Purinergic regulation of the immune system. Nat Rev Immunol 16(3):177–192
Nishimura A, Sunggip C, Oda S, Numaga-Tomita T, Tsuda M, Nishida M (2017) Purinergic P2Y receptors: molecular diversity and implications for treatment of cardiovascular diseases. Pharmacol Ther 180:113–128
Welihinda AA et al (2017) Enhancement of inosine-mediated A 2A R signaling through positive allosteric modulation. Cell Signal 42:227–235
Yarom M et al (1998) Identification of inosine as an endogenous modulator for the benzodiazepine binding site of the GABAA receptors. J Biomed Sci 5(4):274–280
Steinberg GR, Kemp BE (2009) AMPK in health and disease. Physiol Rev 89(3):1025–1078
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13(4):251–262
Kahn BB et al (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1(1):15–25
Ricciarelli R, Fedele E (2018) cAMP, cGMP and amyloid β: three ideal partners for memory formation. Trends Neurosci 41:255–266
Rasmussen H, Kelley G, Douglas JS (1990) Interactions between Ca2+ and cAMP messenger system in regulation of airway smooth muscle contraction. Am J Phys 258(6 Pt 1):L279
Kresge N, Simoni RD, Hill RL (2005) Earl W. Sutherland’s discovery of cyclic adenine monophosphate and the second messenger system. J Biol Chem 19(280):e39
Skalhegg BS, Tasken K (2000) Specificity in the cAMP/PKA signaling pathway. differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci 5(1):D678
Buglioni A, Burnett JC (2016) New Pharmacological Strategies to Increase cGMP. Annu Rev Med 67:229–243
Fan F et al (2016) Age-associated metabolic dysregulation in bone marrow-derived macrophages stimulated with lipopolysaccharide. Sci Rep 6:22637
Ruizgarcía A et al (2011) Cooperation of adenosine with macrophage Toll-4 receptor agonists leads to increased glycolytic flux through the enhanced expression of PFKFB3 gene. J Biol Chem 286(22):19247–19258
Hatfield SM, Sitkovsky M (2016) A2A adenosine receptor antagonists to weaken the hypoxia-HIF-1α driven immunosuppression and improve immunotherapies of cancer. Curr Opin Pharmacol 29:90–96
Akio O et al (2012) The development and immunosuppressive functions of CD4+CD25+FoxP3+regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol 3(3):190–190
Hatfield SM et al (2015) Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci Transl Med 7(277):277ra30
Gnad T et al (2014) Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516(7531):395–399
Ohta A (2016) A metabolic immune checkpoint: adenosine in tumor microenvironment. Front Immunol 7(Pt 1):109
Bantug GR, Galluzzi L, Kroemer G, Hess C (2018) The spectrum of T cell metabolism in health and disease. Nat Rev Immunol 18(1):19–34
Burrows N, Maxwell PH (2017) Hypoxia and B cells. Exp Cell Res 356(2):197–203
Song A, Zhang Y, Han L, Yegutkin GG, Liu H, Sun K, D’Alessandro A, Li J, Karmouty-Quintana H, Iriyama T, Weng T, Zhao S, Wang W, Wu H, Nemkov T, Subudhi AW, Jameson-van Houten S, Julian CG, Lovering AT, Hansen KC, Zhang H, Bogdanov M, Dowhan W, Jin J, Kellems RE, Eltzschig HK, Blackburn M, Roach RC, Xia Y (2017) Erythrocytes retain hypoxic adenosine response for faster acclimatization upon re-ascent. Nat Commun 8:14108
Liu H et al (2016) Adenosine signaling-mediated metabolic reprogramming regulates erythropoiesis. Blood :2437–2437
Palazon A et al (2014) HIF transcription factors, inflammation, and immunity. Immunity 41(4):518–528
Stefania M et al (2005) A3 adenosine receptors modulate hypoxia-inducible factor-1alpha expression in human A375 melanoma cells. Neoplasia 7(10):894–903
Gessi S et al (2013) A 1 and a 3 adenosine receptors inhibit LPS-induced hypoxia-inducible factor-1 accumulation in murine astrocytes. Pharmacol Res 76(10):157–170
Torres A, Erices JI, Sanchez F, Ehrenfeld P, Turchi L, Virolle T, Uribe D, Niechi I, Spichiger C, Rocha JD, Ramirez M, Salazar-Onfray F, San Martín R, Quezada C (2019) Extracellular adenosine promotes cell migration/invasion of glioblastoma stem-like cells through A3 adenosine receptor activation under hypoxia. Cancer Lett 446:112–122
Lagory EL, Giaccia AJ (2016) The ever-expanding role of HIF in tumour and stromal biology. Nat Cell Biol 18(4):356
Fraisl P, Aragonés J, Carmeliet P (2009) Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov 8(2):139
Sitkovsky M, Lukashev D (2005) Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol 5(9):712–721
Xie H, Simon MC (2017) Oxygen availability and metabolic reprogramming in cancer. J Biol Chem 292(41):jbc.R117.799973
Chen CH et al (2001) Regulation of glut1 mRNA by Hypoxia-inducible Factor-1. J Biol Chem 276(12):9519–9525
Borg N, Alter C, Görldt N, Jacoby C, Ding Z, Steckel B, Quast C, Bönner F, Friebe D, Temme S, Flögel U, Schrader J (2017) CD73 on T-cells orchestrates cardiac wound healing after myocardial infarction by purinergic metabolic reprogramming. Circulation 136:297–313
Wang X, Yang K, Xie Q, Wu Q, Mack SC, Shi Y, Kim LJY, Prager BC, Flavahan WA, Liu X, Singer M, Hubert CG, Miller TE, Zhou W, Huang Z, Fang X, Regev A, Suvà ML, Hwang TH, Locasale JW, Bao S, Rich JN (2017) Purine synthesis promotes maintenance of brain tumor initiating cells in glioma. Nat Neurosci 20(5):661–673
Andersson O, Adams BA, Yoo D, Ellis GC, Gut P, Anderson RM, German MS, Stainier DYR (2012) Adenosine signaling promotes regeneration of pancreatic β cells in vivo. Cell Metab 15(6):885–894
Yegutkin GG (2014) Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol 49(6):473–497
Jimenez-Mateos EM, Smith J, Nicke A, Engel T (2018) Regulation of P2X7 receptor expression and function in the brain. Brain Res Bull S0361-9230(18):30734–2
Liu HT, Sabirov RZ, Okada Y (2008) Oxygen-glucose deprivation induces ATP release via maxi-anion channels in astrocytes. Purinergic Signal 4(2):147–154
Hirayama Y, Koizumi S (2017) Hypoxia-independent mechanisms of HIF-1α expression in astrocytes after ischemic preconditioning. Glia 65(3):523–530
Lizhen L et al (2008) Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 28(3):468–481
Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, di Virgilio F (2006) The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176(7):3877–3883
Alessandra P et al (2008) ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A 105(23):8067–8072
Ghiringhelli F, Apetoh LA (2009) Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 15(10):1170–1178
Andrea V et al (2013) Effect of the purinergic inhibitor oxidized ATP in a model of islet allograft rejection. Diabetes 62(5):1665–1675
Vergani A, Tezza S, D’Addio F, Fotino C, Liu K, Niewczas M, Bassi R, Molano RD, Kleffel S, Petrelli A, Soleti A, Ammirati E, Frigerio M, Visner G, Grassi F, Ferrero ME, Corradi D, Abdi R, Ricordi C, Sayegh MH, Pileggi A, Fiorina P (2013) Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation 127(4):463–475
Di VF (2013) The therapeutic potential of modifying inflammasomes and NOD-like receptors. Pharmacol Rev 65(3):872–905
Amoroso F, Capece M, Rotondo A, Cangelosi D, Ferracin M, Franceschini A, Raffaghello L, Pistoia V, Varesio L, Adinolfi E (2015) The P2X7 receptor is a key modulator of the PI3K/GSK3β/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene 34(41):5240–5251
Gómez-Villafuertes R et al (2010) Ca2+/calmodulin-dependent kinase II signalling cascade mediates P2X7 receptor-dependent inhibition of neuritogenesis in neuroblastoma cells. FEBS J 276(18):5307–5325
Beurel E, Michalek SM, Jope RS (2010) Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol 31(1):24–31
Martin M et al (2005) Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol 6(8):777–784
Huang L, Zhang C, Su L, Song Z (2017) GSK3β attenuates TGF-β1 induced epithelial–mesenchymal transition and metabolic alterations in ARPE-19 cells. Biochem Biophys Res Commun 486(3):744–751
Tafani M, de Santis E, Coppola L, Perrone GA, Carnevale I, Russo A, Pucci B, Carpi A, Bizzarri M, Russo MA (2014) Bridging hypoxia, inflammation and estrogen receptors in thyroid cancer progression. Biomed Pharmacother 68(1):1–5
Henrique BDS et al (2018) The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8+ T cells. Nature 559(7713):264–268
Adinolfi E et al (2002) P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood 99(2):706–708
Raffaghello L, Chiozzi P, Falzoni S, di Virgilio F, Pistoia V (2006) The P2X7 receptor sustains the growth of human neuroblastoma cells through a substance P-dependent mechanism. Cancer Res 66(2):907–914
Baricordi OR, Melchiorri L, Adinolfi E, Falzoni S, Chiozzi P, Buell G, di Virgilio F (1999) Increased proliferation rate of lymphoid cells transfected with the P2X(7) ATP receptor. J Biol Chem 274(47):33206–33208
Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, Wieckowski MR, Pinton P, Rizzuto R, di Virgilio F (2005) Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell 16(7):3260–3272
Amoroso F, Falzoni S, Adinolfi E, Ferrari D, di Virgilio F (2012) The P2X7 receptor is a key modulator of aerobic glycolysis. Cell Death Differ 3(8):e370
De Marchi E et al (2019) The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment. Oncogene 38(19):3636–3650
Sara T et al (2012) Extracellular ATP exerts opposite effects on activated and regulatory CD4+ T cells via purinergic P2 receptor activation. J Immunol 189(3):1303–1310
Di VF et al (2017) The P2X7 receptor in infection and inflammation. Immunity 47(1):15
North RA (2016) P2X receptors. Philos Trans R Soc Lond 371(1700):20150427
De ME et al (2016) P2X7 receptor as a therapeutic target. Adv Protein Chem Struct Biol 104:39
Danquah W et al (2016) Nanobodies that block gating of the P2X7 ion channel ameliorate inflammation. Sci Transl Med 8(366):366ra162
Gilbert SM et al (2017) A phase 1 clinical trial demonstrates nfP2X 7 targeted antibodies provide a novel, safe and tolerable topical therapy for BCC. Br J Dermatol 177(1):117–124
Laetitia A et al (2010) Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res 70(3):855–858
Ma Y et al (2013) Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 38(4):729–741
Lecciso M et al (2017) ATP release from chemotherapy-treated dying leukemia cells elicits an immune suppressive effect by increasing regulatory T cells and tolerogenic dendritic cells. Front Immunol 8:1918
Giovanna B et al (2007) Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110(4):1225–1232
Yue N et al (2017) Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J Neuroinflammation 14(1):102
Laskaris LE et al (2016) Microglial activation and progressive brain changes in schizophrenia. Br J Pharmacol 173(4):666–680
Mondelli V et al (2017) Brain microglia in psychiatric disorders. Lancet Psychiatry 4(7):S2215036617301013
Kesteren CFMGV et al (2017) Immune involvement in the pathogenesis of schizophrenia: a meta-analysis on postmortem brain studies. Transl Psychiatry 7(3):e1075
Fischer Y, Becker C, Löken C et al (1999) J Biol Chem 274(2):755–761
Balasubramanian R et al (2014) Enhancement of glucose uptake in mouse skeletal muscle cells and adipocytes by P2Y6 receptor agonists. PLoS One 9(12):e116203–e116203
Hillairebuys D et al (1993) Stimulation of insulin secretion and improvement of glucose tolerance in rat and dog by the P2y-purinoceptor agonist, adenosine-5'-O-(2-thiodiphosphate). Br J Pharmacol 109(1):183–187
Fernandez-Alvarez J, Hillaire-Buys D, Loubatières-Mariani MM, Gomis R, Petit P (2001) P2 receptor agonists stimulate insulin release from human pancreatic islets. Pancreas 22(1):69–71
Hardie DG (2015) AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol 33:1–7
Sebbagh M, Santoni MJ, Hall B, Borg JP, Schwartz MA (2009) Regulation of LKB1/STRAD localization and function by E-cadherin. Curr Biol 19(1):37–42
Chiaranunt P, Ferrara JL, Byersdorfer CA (2015) Rethinking the paradigm: how comparative studies on fatty acid oxidation inform our understanding of T cell metabolism. Mol Immunol 68(2):564–574
Horman S, Beauloye C, Vanoverschelde JL, Bertrand L (2012) AMP-activated protein kinase in the control of cardiac metabolism and remodeling. Curr Heart Fail Rep 9(3):164–173
Steinberg GR, Schertzer JD (2014) AMPK promotes macrophage fatty acid oxidative metabolism to mitigate inflammation: implications for diabetes and cardiovascular disease. Immunol Cell Biol 92(4):340–345
Mihaylova MM, Shaw RJ (2011) The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13(9):1016–1023
Mayer A et al (2008) AMP-activated protein kinase regulates lymphocyte responses to metabolic stress but is largely dispensable for immune cell development and function. Eur J Immunol 38(4):948–956
Xu T, Stewart KM, Wang X, Liu K, Xie M, Ryu JK, Li K, Ma T, Wang H, Ni L, Zhu S, Cao N, Zhu D, Zhang Y, Akassoglou K, Dong C, Driggers EM, Ding S (2017) Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature 548(7666):228–233
Luu M, Pautz S, Kohl V, Singh R, Romero R, Lucas S, Hofmann J, Raifer H, Vachharajani N, Carrascosa LC, Lamp B, Nist A, Stiewe T, Shaul Y, Adhikary T, Zaiss MM, Lauth M, Steinhoff U, Visekruna A (2019) The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat Commun 10(1):760
Julia R et al (2013) AMPKα1: a glucose sensor that controls CD8 T-cell memory. Eur J Immunol 43(4):889–896
Gj VDW et al (2012) Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36(1):68–78
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186(6):3299–3303
Grady SL, Hwang J, Vastag L, Rabinowitz JD, Shenk T (2012) Herpes simplex virus 1 infection activates poly(ADP-ribose) polymerase and triggers the degradation of poly(ADP-ribose) Glycohydrolase. J Virol 86(15):8259–8268
Maciver NJ et al (2011) The liver kinase B1 (LKB1) is a central regulator of T cell development, activation, and metabolism. J Immunol 187(8):4187–4198
Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, Wang Z, Quinn WJ III, Kopinski PK, Wang L, Akimova T, Liu Y, Bhatti TR, Han R, Laskin BL, Baur JA, Blair IA, Wallace DC, Hancock WW, Beier UH (2017) Foxp3 reprograms T cell metabolism to function in low-glucose, High-Lactate Environments. Cell Metab 25(6):1282–1293
Rémi M et al (2013) AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab 18(2):251–264
Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, Tsui YC, Cui G, Micevic G, Perales JC, Kleinstein SH, Abel ED, Insogna KL, Feske S, Locasale JW, Bosenberg MW, Rathmell JC, Kaech SM (2015) Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162(6):1217–1228
Gualdoni GA, Mayer KA, Göschl L, Boucheron N, Ellmeier W, Zlabinger GJ (2016) The AMP analog AICAR modulates the Treg/Th17 axis through enhancement of fatty acid oxidation. FASEB J 30(11):3800–3809
Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186(6):3299–3303
Sereni MI et al (2017) Kinase-driven metabolic signalling as a predictor of response to carboplatin-paclitaxel adjuvant treatment in advanced ovarian cancers. Br J Cancer 117(4):494–502
Song M, Sandoval TA, Chae CS, Chopra S, Tan C, Rutkowski MR, Raundhal M, Chaurio RA, Payne KK, Konrad C, Bettigole SE, Shin HR, Crowley MJP, Cerliani JP, Kossenkov AV, Motorykin I, Zhang S, Manfredi G, Zamarin D, Holcomb K, Rodriguez PC, Rabinovich GA, Conejo-Garcia JR, Glimcher LH, Cubillos-Ruiz JR (2018) IRE1α–XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature 562(7727):423–428
Schworer S, Vardhana SA, Thompson CB (2019) Cancer metabolism drives a stromal regenerative response. Cell Metab 29(3):576–591
Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA, Kimmelman AC (2016) Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536(7617):479–483
Katheder NS, Khezri R, O’Farrell F, Schultz SW, Jain A, Rahman MM, Schink KO, Theodossiou TA, Johansen T, Juhász G, Bilder D, Brech A, Stenmark H, Rusten TE (2017) Microenvironmental autophagy promotes tumour growth. Nature 541(7637):417–420
Yang Z et al (2017) ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol Cancer 16(1):79
Lin L, Huang H, Liao W, Ma H, Liu J, Wang L, Huang N, Liao Y, Liao W (2015) MACC1 supports human gastric cancer growth under metabolic stress by enhancing the Warburg effect. Oncogene 34(21):2700–2710
Yang T et al (2016) MACC1 mediates acetylcholine-induced invasion and migration by human gastric cancer cells. Oncotarget 7(14):18085–18094
Kubben N, Misteli T (2017) Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat Rev Mol Cell Biol 18(10):595–609
Cantó C et al (2010) Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 11(3):213–219
Garciaroves PM et al (2008) Gain-of-function R225Q mutation in AMP-activated protein kinase gamma3 subunit increases mitochondrial biogenesis in glycolytic skeletal muscle. J Biol Chem 283(51):35724–35734
Maniam S et al (2015) Cofactor strap regulates oxidative phosphorylation and mitochondrial p53 activity through ATP synthase. Cell Death Differ 22(1):156–163
Sanchezmacedo N et al (2013) Depletion of the novel p53-target gene carnitine palmitoyltransferase 1C delays tumor growth in the neurofibromatosis type I tumor model. Cell Death Differ 20(4):659–668
Klingenberg M (1990) Mechanism and evolution of the uncoupling protein of brown adipose tissue. Trends Biochem Sci 15(3):108–12
Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD (2002) Superoxide activates mitochondrial uncoupling proteins. Nature 415(6867):96–99
Claire P et al (2008) Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. FASEB J 22(1):9–18
Alexandra K et al (2014) Loss of UCP2 attenuates mitochondrial dysfunction without altering ROS production and uncoupling activity. PLoS Genet 10(6):e1004385
Jin Z et al (2014) UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J 30(24):4860–4873
Vozza A, Parisi G, de Leonardis F, Lasorsa FM, Castegna A, Amorese D, Marmo R, Calcagnile VM, Palmieri L, Ricquier D, Paradies E, Scarcia P, Palmieri F, Bouillaud F, Fiermonte G (2014) UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci U S A 111(3):960–965
Zhonglin X et al (2008) Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes 57(12):3222–3230
Marc F et al (2005) Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes 54(5):1331–1339
Pauline E et al (2014) Mitochondrial retrograde signaling mediated by UCP2 inhibits cancer cell proliferation and tumorigenesis. Cancer Res 74(14):3971–3982
Wang M et al (2017) Uncoupling protein 2 downregulation by hypoxia through repression of peroxisome proliferator-activated receptor γ promotes chemoresistance of non-small cell lung cancer. Oncotarget 8(5):8083
Boutoual R et al (2018) Defects in the mitochondrial-tRNA modification enzymes MTO1 and GTPBP3 promote different metabolic reprogramming through a HIF-PPARγ-UCP2-AMPK axis. Sci Rep 8(1):1163
Szelechowski M et al (2018) Metabolic reprogramming in amyotrophic lateral sclerosis. Sci Rep 8(1):3953
Daurio NA et al (2016) AMPK activation and metabolic reprogramming by tamoxifen through estrogen receptor-independent mechanisms suggests new uses for this therapeutic modality in cancer treatment. Cancer Res 76(11):3295
Pulito C, Mori F, Sacconi A, Goeman F, Ferraiuolo M, Pasanisi P, Campagnoli C, Berrino F, Fanciulli M, Ford RJ, Levrero M, Pediconi N, Ciuffreda L, Milella M, Steinberg GR, Cioce M, Muti P, Strano S, Blandino G (2017) Metformin-induced ablation of microRNA 21-5p releases Sestrin-1 and CAB39L antitumoral activities. Cell Discov 3:17022
Lodi A, Woods SM, Ronen SM (2014) Magnetic resonance-detectable metabolic consequences of MEK inhibition. NMR Biomed 27(6):700
Oliveras-Ferraros C, Vazquez-Martin A, Cuyàs E, COROMINAS-FAJA B, Rodríguez-Gallego E, Fernández-Arroyo S, Martin-Castillo B, Joven J, MENENDEZ MENENDEZ J (2014) Acquired resistance to metformin in breast cancer cells triggers transcriptome reprogramming toward a degradome-related metastatic stem-like profile. Cell Cycle 13(7):1132–1144
Zhou X et al (2016) Metformin suppresses hypoxia-induced stabilization of HIF-1α through reprogramming of oxygen metabolism in hepatocellular carcinoma. Oncotarget 7(1):873–884
Zarrouk M et al (2014) Adenosine-mono-phosphate-activated protein kinase-independent effects of metformin in T cells. PLoS One 9(9):e106710
Hardie DG (2007) AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol 47(1):185–210
Popovics P, Frigo DE, Schally AV, Rick FG (2015) Targeting the 5'-AMP-activated protein kinase and related metabolic pathways for the treatment of prostate cancer. Expert Opin Ther Targets 19(5):617–632
Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444(7117):337–342
Gerhart-Hines Z et al (2011) The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD +. Mol Cell 44(6):851–863
Maus M, Cuk M, Patel B, Lian J, Ouimet M, Kaufmann U, Yang J, Horvath R, Hornig-Do HT, Chrzanowska-Lightowlers ZM, Moore KJ, Cuervo AM, Feske S (2017) Store-operated Ca2+ entry controls induction of lipolysis and the transcriptional reprogramming to lipid metabolism. Cell Metab 25(3):698–712
Gong KW et al (2001) cAMP-specific phosphodiesterase TbPDE1 is not essential in Trypanosoma brucei in culture or during midgut infection of tsetse flies. Mol Biochem Parasitol 116(2):229
Cheng KK, Lee BS, Masuda T, Ito T, Ikeda K, Hirayama A, Deng L, Dong J, Shimizu K, Soga T, Tomita M, Palsson BO, Robert M (2014) Global metabolic network reorganization by adaptive mutations allows fast growth of Escherichia coli on glycerol. Nat Commun 5(1):3233
Lu J et al (2017) Corticotropin releasing hormone can selectively stimulate glucose uptake in corticotropinoma via glucose transporter 1. Mol Cell Endocrinol 470:105–114
Tong T, Ryu SE, Min Y, de March CA, Bushdid C, Golebiowski J, Moon C, Park T (2017) Olfactory receptor 10J5 responding to α-cedrene regulates hepatic steatosis via the cAMP–PKA pathway. Sci Rep 7(1):9471
Nissim I et al (2014) The molecular and metabolic influence of long term agmatine consumption. J Biol Chem 289(14):9710–9729
Carlessi R, Chen Y, Rowlands J, Cruzat VF, Keane KN, Egan L, Mamotte C, Stokes R, Gunton JE, Bittencourt PIH, Newsholme P (2017) GLP-1 receptor signalling promotes β-cell glucose metabolismviamTOR-dependent HIF-1α activation. Sci Rep 7(1):2661
Funding
This work was financially supported by the grants from the Health and Family Planning Commission of Hunan Province (B20180855), Innovation Driven Planning of Central South University(2018CX028), and High-level Talent Planning of Xiangya Hospital.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human participants and/or animals
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, Z., Ye, W., Chen, H. et al. Role of purines in regulation of metabolic reprogramming. Purinergic Signalling 15, 423–438 (2019). https://doi.org/10.1007/s11302-019-09676-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-019-09676-z