Abstract
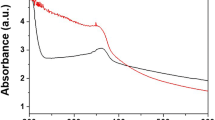
Zinc deficiency in aquatic animals affects the biological processes and physiological functions. Thus, the supplement of ZnONPs can be used as an alternative method to overcome zinc deficiency. Nanoparticles have the potential to enhance the growth and health of the fish. The main aim of this study is to evaluate the growth efficacy of ZnONP-supplemented diet with fingerlings of Labeo rohita. The green synthesized ZnONPs were characterized by ultraviolet-visible (UV-Vis) spectroscopy, Fourier transformer infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive spectroscopy (EDS). Different concentrations of ZnONPs (5, 7.5, and 10 mg/kg) were administered in the basal diet of freshwater fish Labeo rohita for 45 days to observe the growth and metabolic functions of the body. However, the fish fed with 10 mg/kg ZnONP-supplemented diet shows that the growth performance was highly increased followed by 7.5 mg/kg and 5 mg/kg ZnONPs when compared with the control. The biochemical, hematological, and digestive enzyme activities were also significantly increased with different concentrations of ZnONPs. The effects of zinc oxide nanoparticles show the higher improvement of growth and metabolic functions in Labeo rohita. These results suggest that the nanotechnology could apply for feed formulation technology and pave the way for the dietary supplementation of zinc oxide nanoparticles as safe ingredients for aquatic animals to overcome the zinc deficiency.









Similar content being viewed by others
Abbreviations
- ANOVA:
-
analysis of variance
- DMRT :
-
Duncan’s Multiple Range test
- EDS :
-
energy dispersive spectroscopy
- FTIR :
-
Fourier transformer infrared spectroscopy
- SEM :
-
scanning electron microscopy
- XRD :
-
X-ray diffraction pattern
- ZnONPs :
-
zinc oxide nanoparticles
References
Muralisankar T, Saravana Bhavan P, Radhakrishnan S, Seenivasan C, Srinivasan V, Santhanam P (2014) Effects of dietary zinc on the growth, digestive enzyme activities, muscle biochemical compositions and antioxidant status of the giant freshwater prawn Macrobrachium rosenbergii. Biol Trace Elem Res 160(1):56–66
Bharadwaj AS, Patnaik S, Browdy CL, Lawrence L (2016) Availability of dietary zinc sources and effects on performance of pacific white shrimp Litopenaeus vannamei (Boone). Int J Recirculating Aquac 13:1–10. https://doi.org/10.21061/ijra.v13i0.1514
Subcommittee on Fish nutrition, National Research Council (1993) Nutrient requirements of fish. Washington, DC: The National Academies Press, Page no. 20. https://doi.org/10.17226/2115
Rather MA, Sharma R, Aklakur M, Ahmad S, Kumar N, Khan M, Ramya VL (2011) Nanotechnology, a novel tool for aquaculture and fisheries development a prospective mini-review. Fish Aquac J 16:1–6
Hamed M, Darroudi M (2017) Zinc oxide nanoparticles: biological synthesis and biomedical applications. Ceram Int 43(1):907–914
Khan I, Saeed K, Khan I (2017) Nanoparticles: properties, applications and toxicities. Arab J Chem https://doi.org/10.1016/j.arabjc.2017.05.011
Krithika G, Saraswathy R, Muralidhar M, Thulasi D, Lalitha N, Kumararaja P, Nagavel A, Balaji A, Jayavel R (2017) Zinc oxide nanoparticles-synthesis, characterization and antibacterial activity. J Nanosci Nanotechnol 17(8):5209–5216
Umalatha, Sridhar N, Kushwaha JP, Gangadhar B (2016) Digestive enzyme activities in different size groups and segments of the digestive tract in Labeo rohita (Day, 1878). J Aquacult Mar Biol 4(5):00098
Elumalai K, Velmurugan S (2015) Green synthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from the leaf extract of Azadirachta indica (L.). Appl Surf Sci 345(22):329–336
Tamanna B, Kavita M, Manika K, Prasad R, Varma A (2015) Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process 32:55–61
Olasupo AD, Aborisade AB, Olagoke OV (2018) Phytochemical analysis and antibacterial activities of Spinach leaf. Am J Phytomed Clin Ther 2(2):8. https://doi.org/10.21767/2321-2748.100344
Soni and Sosa (2013) Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J Pharmacogn Phytother 2(4):22–29
Debnath D, Pal AK, Sahu NP, Yengkokpam S, Baruah K, Choudhury D, Venkateshwarlu G (2007) Digestive enzymes and metabolic profile of Labeo rohita fingerlings fed diets with different crude protein levels. Comp Biochem Physiol B Biochem Mol Biol 146(1):107–114
Lakshmi, Bai RS, Sharanagouda H, Ramachandra CT, Sushila N, Doddagoudar SR (2017) Biosynthesis and characterization of ZnO nanoparticles from Spinach (Spinacia oleracea) leaves and its effect on seed quality parameters of green gram (Vigna radiata). Int J Curr Microbiol App Sci 6(9):3376–3384
Evans WC (1989) Trease and Evans pharmacognosy. Balliere Tindall, London, pp 216–217
Uddin MN, Alam SN, Mobin MN, Miah MA (2014) An assessment of the river water quality parameters: a case of Jamuna River. J Environ Sci Nat Res 7(1):249–256
Tapati D, Bipasha DG, Narayan DD (2016) Formulation of low-cost fish feed using locally available agro-based wastes and its efficacy in growth performance of common carp (Cyprinus carpio L.) – a case study from Apatani landscape of Arunachal Pradesh in Northeast India. Int Res J Biol Sci 5(3):61–67
Onuegbu CU, Agarwal A, Singh NB (2018) ZnO nanoparticles as feed supplement on growth performance of cultured African catfish fingerlings. J Sci Ind Res 77(4):213–218
Annamalai A, Bhavan PS, Vimala K, Karthik M, Cheruparambath P (2016) Dietary supplementation of green synthesized manganese oxide nanoparticles and its effect on growth performance, muscle composition and digestive enzyme activities of the giant freshwater prawn Macrobrachium rosenbergii. J Trace Elem Med Biol 35(3):7–17
Folch J, Lees M, Bloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 266(1):497–509
Barnes H, Black Stock J (1973) Estimation of lipids in marine animals and tissues detailed investigation of the sulpho phosphor vanillin method for total lipids. J Exp Mar Biol Ecol 12(1):103–118
Remya AS, Ramesh M, Saravanan M, Poopal RK, Bharathi S, Natraj D (2014) Iron oxide nanoparticles to an Indian major carp, Labeo rohita: impacts on hematology, iono regulation and gill Na+ /K+ ATPase activity. J King Saud Univ- Sci 27(2):151–160
Behera T, Swain P, Rangacharulu PV, Samanta M (2014) Nano-Fe as feed additive improves the hematological and immunological parameters of fish, Labeo rohita H. Appl Nanosci 4(6):687–694
Drabkin DL (1950) The distribution of the chromoproteins, hemoglobin, myoglobin and cytochrome C, in the tissues of different species, and the relationship of the total content of each chromoprotein to body mass. J Biol Chem 182(1):317–333
Dacie V, Lewis SM (1991) Practical hematology, 7th edn. Churchill Livingstone, London, p 556
Stoskopf MK (1993) Fish medicine. W B Saunders Company, Philadelphia
Furne M, Hidalgo MC, Lopez A, Garcia-Gallego M, Morales AE, Domenzain A, Domezain J, Sanz A (2005) Digestive enzyme activities in Adriatic sturgeon Acipenser naccarii and rainbow trout Oncorhynchus mykiss. A comparative study. Aquaculture 250(1–2):391–398
Bernfeld P (1955) Amylases. In: Colowick SP, Kaplan NO (eds) Methods in enzymology. Academic Press, New York, pp 149–158
Swain P, Das R, Das A, Padhi SK, Das KC, Mishra SS (2018) Effects of dietary zinc oxide and selenium nanoparticles on growth performance, immune responses and enzyme activity in rohu, Labeo rohita (Hamilton). Aquac Nutr 27(3):1–9
Vanathi P, Rajiv P, Narendhran SS, Rajeshwari S, Rahman PKSM, Venckatesh R (2014) Biosynthesis and characterization of phytomediated zinc oxide nanoparticles: a green chemistry approach. Mater Lett 134(9):13–15
Elizabeth V, Mary G (2015) Green synthesis of zinc oxide nanoparticles. Int J Adv Res Sci Eng 4(1):307–314
Sachudanandam N, Shakila D, Geetha K, Dinesh Karthik A (2017) Novel green synthesis of zinc oxide nanoparticles & study of its in vitro antimicrobial activity. IOSR J Pharm 2:01–06
Sangeetha G, Rajeshwari S, Venckatesh R (2011) Green synthesis of zinc oxide nanoparticles by Aloe barbadensis miller leaf extract: structure and optical properties. Mater Res Bull 46(12):2560–2566
Gnanasangeetha D, Sarala TD (2013) One pot synthesis of zinc oxide nanoparticles via chemical and green method. Res J Mater Sci 1(7):1–8
Salam HA, Rajeshwari S, Venkatesh R (2014) Green synthesis and characterization of zinc oxide nanoparticles from Ocimum basilicum L. var. purpurascens Benth-LAMIACEAE leaf extract. Mater Lett 131:16–18
Handy RD (2012) FSBI Briefing paper: nanotechnology in fisheries and aquaculture. Fisheries Society of the British Isles, 1–29
Tawfik MMM, Moustafa MM, Abumourad IMK, EI-Meliegy EM, Refai MK (2017) Evaluation of nano zinc oxide feed additive on tilapia growth and immunity. In 15th International Conference on Environmental Science and Technology, Rhodes, Greece, 1342:1
Acknowledgments
The authors greatly acknowledge the Department of Zoology, PSG College of Arts and Science, for the facilities extended towards the research.
Availability of Data and Materials
All the data and materials presented in the manuscript are the original work of the authors.
Author information
Authors and Affiliations
Contributions
SM performed the experiment. SM has written the manuscript. ST was sourced as a research supervisor and designed the work. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethical Approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thangapandiyan, S., Monika, S. Green Synthesized Zinc Oxide Nanoparticles as Feed Additives to Improve Growth, Biochemical, and Hematological Parameters in Freshwater Fish Labeo rohita. Biol Trace Elem Res 195, 636–647 (2020). https://doi.org/10.1007/s12011-019-01873-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01873-6