Abstract
Effective drug delivery to the CNS to achieve the desired therapeutic response is a significant challenge in the field of drug delivery. In central nervous system (CNS), blood brain barrier (BBB) restricts the desired therapeutic responses due to inefficient targeting, release kinetics, and failure to reach therapeutic concentrations in the brain. Therefore, most potentially beneficial diagnostic and therapeutic agents are not able to reach to the brain upon systemic administration. Despite the existence of many invasive techniques to promote drug deliveries across BBB, novel strategies of drug delivery system which can cross BBB effectively are required, otherwise translation of novel neurotherapeutics from bench to bedside will be difficult to achieve. In this review, we briefly outline the existing and emerging strategies for CNS drug deliveries with a focus on potential and challenges of using extracellular vesicles (EVs) in CNS drug delivery system. EVs are emerging as a promising tool for therapeutic delivery owing to its favorable intrinsic features of biocompatibility, stability, stealth capacity, ability to overcome natural barriers and inherent homing capability. EVs are nanovesicles that allow cell-cell communication. The EVs-cargo reflects the physiological as well as the pathophysiological state of a cell. EVs are shown to play a role in human immunodeficiency virus (HIV) infection and dissemination, which contributes to acquired immune deficiency syndrome (AIDS). In the context of HIV-1 infection, this review also outlines the role of EVs in dissemination, challenges faced in EVs research in HIV-1 co-morbid conditions and potential of nanotechnologies, especially EVs in Neuro-AIDS.

EVs are used for the delivery of small molecule drugs, protein, and nucleic acid to the CNS as well as imaging molecules for in vivo tracking. For the purpose of delivery, EVs may or may not be subjected to membrane modification. The advantages of EVs, including its biocompatibility, low immunogenicity, and low toxicity profiles, can be exploited to potentially devise novel therapeutic delivery system for CNS drug targeting. This article outlines the challenges in potential EV-based therapeutic delivery.
Similar content being viewed by others
Introduction
Search for novel drug delivery systems and improvement of the existing ones for better pharmacokinetics and enhanced ability to carry drugs across the central nervous system (CNS) is a vital area of research for many researchers. Proteins, nucleic acids, and other macromolecules pose challenge of crossing the blood-brain barrier (BBB) when intended to be used as CNS therapeutics (Wong et al. 2012). In CNS, BBB restricts the desired therapeutic responses due to inefficient targeting, release kinetics, and failure to reach therapeutic concentrations in the brain (Druzhkova and Yakovlev 2018). Therefore, most potentially beneficial diagnostic and therapeutic agents are not able to reach to the brain upon systemic administration (Almutairi et al. 2016; Marianecci et al. 2017; Pardridge 2012). Many invasive techniques such as neurosurgery and osmotic/biochemical opening of the BBB have been used for drug delivery to the brain, which implies a reduction in drug efficacy and increase risks to patients (Abbott et al. 2010). Until we have a drug delivery system which can cross BBB effectively, translation of novel neurotherapeutics from bench to bedside will be difficult to achieve.
Discovery of extracellular vesicles (EVs) as key players in cell-cell communication has not only demonstrated the ability of EVs to serve as potential biomarkers of diseases (Kodidela et al. 2019; Pulliam et al. 2019), but also, delineated their role as potential drug delivery vehicles (Shah et al. 2018). EVs are emerging as a promising tool for therapeutic delivery owing to its favorable intrinsic features of biocompatibility, stability, stealth capacity, ability to overcome natural barriers and inherent homing capability (Rufino-Ramos et al. 2017). EVs, which are heterogeneous lipid bilayer membrane-enclosed vesicles (30–1000 nm) are secreted ubiquitously by several cell types into the extracellular microenvironment (Antonyak and Cerione 2015; Shah et al. 2018). The packaged cargo within the EVs reflects the physiological as well as the pathophysiological state of the cell of origin (Shah et al. 2018). The lipid bilayer protects the luminal cargo of EVs from the strident environmental conditions (Mathieu et al. 2019). EVs may have different physiological functions based on cellular origin, such as tissue repair and regeneration (Nawaz et al. 2018; van Poll et al. 2008) anti-inflammation/immunomodulatory (Pacienza et al. 2019; Tan et al. 2016), cytoprotective (Xin et al. 2013a), and many other functions as outlined by a paper from International Society of Extracellular Vesicles (ISEV)(Yáñez-Mó et al. 2015). Both in physiological as well as neurodegenerative and neuroinflammatory disease states of CNS, EVs are recognized as an important modulator in cross-talking between neurons, astrocytes, microglia, and oligodendrocytes (Rufino-Ramos et al. 2017).
Over a period of time, these secreted vesicles have been given different names, but today they are collectively referred to as EVs (Mathieu et al. 2019); and this is the term we will use widely in this review. Based on size and their biogenesis, EVs have been distinctly divided into three main subcategories; exosome (30–150 nm), microvesicles (100–1000 nm), and apoptotic bodies (500–5000 nm), and have been shown to play an important role in human immunodeficiency virus (HIV) infection and dissemination (Schorey et al. 2015). Several studies have been conducted to engineer EVs (Gilligan and Dwyer 2017; Luan et al. 2017) as refined biological nanoplatforms for drug delivery to CNS as shown in Table 1. Despite the suggestive evidence that EVs can cross BBB, there is a limited understanding of the trafficking mechanism of EVs under physiological and pathological conditions. Further understanding of the mechanism is essential for successful implementation of EVs as therapeutic drug delivery systems to CNS.
In this review, we briefly outline the existing and emerging strategies for CNS drug deliveries with a focus on potential and challenges of using EVs in CNS drug delivery system. In the context of HIV-1 infection, we also outlined the role of EVs in dissemination, challenges faced in EVs research in HIV-1 co-morbid conditions and potential of nanotechnologies, especially EVs in Neuro-AIDS.
Existing Strategies for CNS Drug Deliveries
The BBB offers trans-endothelial resistance to the passage of endogenous molecules, xenobiotics, and immune surveilling cells such as macrophages, therefore, maintains CNS homeostasis (Zhou et al. 2018). Apart from the physical barriers, metabolism-driven barriers also restrict the passage of drugs across the BBB either by metabolic degradation or drug efflux. For example, functional ATP- binding cassette (ABC) drug efflux transporters, expressed on glial cells, have shown to alter drug uptake and distribution of anti-retroviral drugs, therefore acting as a secondary barrier to drug penetration (van Tellingen et al. 2015). To meet the challenges of drug penetration in the brain, several advancements have been made to improve paracellular and transcellular drug delivery methods (Hersh et al. 2016). Preclinical studies using temporary disruption of BBB by osmotic pressure demonstrated improved chemotherapeutic agent delivery with methotrexate in Phase I clinical trial (Neuwelt et al. 1980). However, neurotoxic levels of blood protein accumulation and transient cerebral edema due to non-specific BBB disruption were critical limitations (Hersh et al. 2016). Other studies have shown that chemically modifying drugs into prodrugs enhances their lipophilic nature, triggering receptor-mediated transcytosis (RMT), thereby, enhancing drug delivery across BBB (Hersh et al. 2016). This approach has been proven to be successful in a recent study that utilizes insulin receptor-targeted antibody and therapeutic protein fusion for treating mucopolysaccharidosis type I (MPS-I) and AD in non-human primates (Mäger et al. 2017). Alternative strategies, such as invasive neurosurgery for local drug administration or non-invasive temporary disruption of BBB by microbubble-enhanced focused ultrasound are risky procedures because such invasive procedures can often compromise the BBB integrity thereby allowing passage of toxic endogenous or exogenous compounds into the brain (Arvanitis and McDannold 2015).
Viral vectors were also investigated for their ability to deliver therapeutic genes (Fu and McCarty 2016; Hersh et al. 2016). There are several limitations to using viral vectors, such as immunogenicity, lack of systemic delivery technologies for CNS, poor BBB passage, the dependence of the spread of the viral vectors in target tissue on replication-competency and target specificity (Murphy and Rabkin 2013; Upadhyay 2014). Interestingly, studies have been conducted using viral derived peptides, such as Rabies viral glycoprotein (RVG), as targeting ligands to the brain. RVG is a viral component that has an inherent neurotrophic nature making it a promising tool for an effective and non-invasive mode for overcoming the BBB (Huey et al. 2017). Therefore, it is possible to exploit RVG’s brain targeting capacity to deliver therapeutic agents to the brain (Alvarez-Erviti et al. 2011).
Emerging Strategies for CNS Drug Deliveries across BBB
With the recent advancements in nanotechnology, nanocarrier based drug delivery systems are now available to allow drug delivery across the BBB. Owing to their small size and enhanced solubility, the rationale for using nanocarriers include site-specific drug targeting, reduced side effects, controlled drug release profile, and favorable drug pharmacokinetics (Hersh et al. 2016). Nanoparticles (polymeric nanoparticle, solid lipid nanoparticle, lipid nanocapsule, and albumin nanoparticle) and other nanocarriers such as liposome, micelle, dendrimer, nanogel, nanoemulsion, and nanosuspension have been described as favorable systems for CNS drug delivery (Wong et al. 2012).
Liposomal and Polymeric Drug Delivery Systems
In many biomedical areas, use of liposomal and polymeric drug delivery systems had great impact on delivering drugs such as anti-retroviral (ARV) drugs across the BBB (Duncan and Gaspar 2011; Nair et al. 2016). Polymeric nanoparticles allow drugs to be associated or encapsulated with natural or synthetic polymers that impart stability, improved in vivo pharmacokinetics, and controlled release of drugs (Hersh et al. 2016). Despite the capability for large scale production of high-quality polymers and ability to modify functional and biodegradable properties, certain limitations exist. Most common limitations are batch-to-batch variability, reduced half-life in systemic circulation, immunogenicity, inefficiency to cross the BBB, and insufficient biocompatibility and toxicological data (Gabathuler 2010; Lanone and Boczkowski 2006; Moghimi et al. 2005; Nair and Laurencin 2006).
Lipid-based nanocarriers of which liposomes are the most commonly used (Mishra et al. 2018), have some disadvantages that are common to the polymeric systems owing to the non-endogenous origin, as shown in Table 2. Despite being able to confer encapsulation and protection of both hydrophilic and lipophilic drugs and lower off-target side effects, the low stability upon systemic injection and retention by off-target organs such as liver, lungs, and spleen poses a significant challenge for efficient delivery of therapeutic concentrations to the target site (Johnston et al. 2007; Takeuchi et al. 2000).
EVs Drug Delivery System
EVs are released by most of the cells both in the periphery and in the CNS. EV-associated cargo, such as proteins, lipids, and RNA, is mostly dependent on the donor cells they originate from and are crucial to maintaining physiological homeostasis. EVs are also shown to be involved in pathological conditions, such as autoimmune disorder (Katsiougiannis 2015), cancer (Rajagopal and Harikumar 2018; Zhang et al. 2017), cardiovascular diseases (Zhang et al. 2017), and infectious and neurodegenerative diseases (Jan et al. 2017; Quek and Hill 2017; Shah et al. 2018). Recently, many studies have successfully described EVs as promising tools for diagnostic and drug delivery systems. In ischemic stroke models, strategies have been devised to graft mesenchymal stem cells (MSCs) to induce brain remodeling via paracrine effects of the MSC secretome, including, EVs (Zagrean et al. 2018). In ischemic brain tissue, restorative effects of stem and progenitor cells have been reported to be the consequence of paracrine mechanisms of grafted cells instead of cell regeneration, where EVs are thought to play a key role (Doeppner et al. 2018). This study highlights the implications of using stem cell derived EVs to act as natural nanocarriers to deliver active biomolecules across the BBB.
EVs have several desirable features owing to its endogenous origin (Abels and Breakefield 2016) that include its biocompatibility, inherent targeting capacity, low immunogenicity, and low toxicity profiles (Turturici et al. 2014). Exosomes, a class of EVs, have also been reported to be able to carry small molecules across the BBB, thus improving the drug transport to the brain (Didiot et al. 2016). Considering the benefits of using EVs, research has intensified to exploit EVs as a drug delivery vehicle. Previous reports have revealed that EVs can be engineered to modify its surface properties to carry the loaded exogenous cargo upon systemic administration as discussed in the later sections. However, there is a limited understanding of the mechanism of EVs transport across BBB under physiological and pathological conditions (Chen et al. 2016; Matsumoto et al. 2017). Also, some reported that surface modified EVs carry its cargo across BBB via receptor-mediated endocytosis (Tian et al. 2018; Yang et al. 2015).
In the hope for overcoming hurdles of CNS drug delivery across the BBB, many researchers have engineered/modified EVs to exploit them as a tool for drug delivery as summarized in Table 1.
EVs as a Vehicle for Small Molecules
EV encapsulation has been shown to enhance properties of drugs apart from being able to carry them across the BBB. In a lipopolysaccharide (LPS)-induced septic shock mouse model, the exosome encapsulated anti-inflammatory agent curcumin was shown to be more stable and highly concentrated in the blood. Improvement of curcumin activity was assessed both by in vitro and in vivo assays upon curcumin administration, with or without encapsulated exosome. Exosome encapsulated curcumin showed improved activity in both in vitro and in vivo cases (Sun et al. 2010). Another study showed that upon intranasal administration, exosome encapsulated curcumin, or a signal transducer and activator of transcription 3 (Stat3) inhibitor was successfully and rapidly delivered to the microglial cells to induce apoptosis. The results showed protection from LPS-induced brain inflammation and progression of myelin oligodendrocyte glycoprotein (MOG) peptide-induced experimental autoimmune encephalomyelitis (EAE), and also showed significantly delayed brain tumor growth in the GL26 tumor model (Zhuang et al. 2011).
There has also been considerable research done to encapsulate anti-cancer drugs into EVs (You et al. 2018). BBB restricts the penetration of anticancer drugs such as methotrexate, paclitaxel, doxorubicin, and vincristine (Yang et al. 2015). Interestingly, brain endothelial cell-derived exosomes were shown to deliver anticancer drugs paclitaxel and doxorubicin across the BBB for the treatment of brain cancer in a zebrafish (Danio rerio) model, revealing the potential to use EVs as carriers for delivery of drugs for brain cancers (Yang et al. 2015).
EVs as a Vehicle for Nucleic Acids
Munoz et al. have reported the use of MSC-derived EVs to deliver small interfering RNA (siRNA) against miR-9 (Munoz et al. 2013). miR-9 is involved in the expression of the drug efflux protein P-glycoprotein, which contributes to drug resistance. Delivery of EV-encapsulated anti-miR-9 to the Glioblastoma Multiforme (GBM) cells was shown to reduce the expression of the efflux transporter and improve the sensitivity of GBM cells to Temozolomide (TMZ). Loading EVs directly with TMZ, an agent used to treat GBM, may also be beneficial to overcome the issues of chemoresistance (Gourlay et al. 2017).
A study by Alvarez-Erviti et al. (Alvarez-Erviti et al. 2011) showed for the first time that engineered EVs can penetrate the BBB and efficiently be used for brain delivery. They used dendritic cell (DC)-derived lysosome-associated membrane protein (Lamp2b) expressing exosomes fused to the RVG peptide. RVG peptide allows targeting to neurons and brain endothelial cells to promote BBB crossing. Exosomes were loaded with siRNA for housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and beta-secretase 1(BACE1), a therapeutic target for Alzheimer’s disease (AD). Significant knockdown of both mRNA, as well as protein levels, was achieved in neurons, microglia, and oligodendrocytes in an AD mouse model. This study for the first time highlighted the potential use of exosomes as delivery tools to silence genes relevant in neurodegenerative diseases. Using similar techniques in a transgenic mouse model for Parkinson’s disease (PD), DC-derived exosomes were used to deliver siRNA for α-synuclein (α-syn), a characteristic protein aggregate in PD, in an attempt to reverse or delay the Parkinsonian symptoms (Cooper et al. 2014). Results showed successful ablation of the mRNA and protein levels in both normal and S129D α-Syn transgenic mice. Similar strategies were also employed to deliver exosome encapsulated hydrophobically modified siRNAs (hsiRNAs) targeting Huntingtin mRNA in mouse models, resulting in lowered Huntingtin mRNA and protein levels, thus suggesting the implications of using EVs as vehicles in treatment of neurological diseases (Didiot et al. 2016).
EVs as a Vehicle for Proteins
Apart from delivery of small molecules and nucleic acids, EVs have also been exploited to deliver therapeutic proteins to the brain. Progression of PD involves brain inflammation, microglial activation, and oxidative stress, which are implicated in the neurodegeneration. Catalase, a natural antioxidant, has been reported to rescue primary cerebellar granule cells in in vitro PD models. Due to the poor penetration of catalase and rapid degradation upon systemic administration, Haney et al. investigated the effectiveness of EVs loaded with catalase (Haney et al. 2015). It was reported that catalase loaded exosomes protect the substantia nigra pars compacta (SNpc) neurons against oxidative stress in mice with acute brain inflammation.
EVs as a Vehicle for Imaging Molecules
To monitor the biodistribution and levels of circulating EVs, it is advantageous to perform EV surface modification or to load cargo such that, upon systemic administration, they can be tracked. Bioluminescence-mediated tomography imaging on mice, injected with human embryonic kidney 293 T (HEK293T) EVs expressing a membrane-bound Gaussian luciferase-biotin-fluorescent streptavidin complex, was done to monitor systemic localization of exosomes (Lai et al. 2014). Antibodies against the EV proteins CD24 and aquaporin 2 (AQP2) conjugated to a fluorophore were used to identify EV-subpopulations that express CD24 and AQP2 by nanoparticle tracking analysis (NTA) (Oosthuyzen et al. 2013). Use of multimodal decorated nanoparticles, such as europium (Eu3+)- doped cobalt ferrite, to load EVs can be further explored for rapid and real-time in vivo tracking of EVs distribution via magnetic resonance imaging (MRI). Eu3+ component enables fluorescence imaging for histological validation of cell localizations, and cobalt ferrite confers magnetic sensitivity to the nanoparticles enabling them to be scanned by MRI (Kevadiya et al. 2018).
Despite the prospects of using EVs as drug delivery vehicles, there exist many challenges, which are discussed in the following section, necessitating extensive investigations.
Challenges and Future of EV-Based Drug Delivery
Isolation and Characterization of EVs
To study the characteristics and application of EVs as drug delivery vehicles, EVs must be efficiently isolated in sufficient quantity from various sources (either from cell culture or biofluids) and should be free from cellular and molecular contaminants. The surface and cargo content of the EVs require careful characterization to conclusively define the cargo repertoire and role of the EVs. Since there exists an ongoing debate as to whether the EV-number, amount of EV-content or ratio of EV-number to the amount of EV-content should be used to quantify EVs (Lv et al. 2018), in a clinical context, standardization of EVs quantification is critical to define the EV-dosage to be used.
Production of EVs
Potential of EVs to be used as therapeutic carriers depend on the ability to produce EVs in a large-scale (Yamashita et al. 2018). Table 3 outlines the potential advantages and disadvantages of the currently existing methods of EV-isolation. Efficient isolation techniques for EVs can aid in increased yield. Despite the progress made in EV-isolation techniques, efficient isolation of EVs has been complicated due to the complexity of the biological samples, heterogeneity of the EVs, and overlap of the biological and physicochemical properties (Willis et al. 2017). The techniques available for isolation of EVs may co-purify different subpopulations of EVs and other particles or soluble factors (Webber and Clayton 2013; Willis et al. 2017). Ramirez et al. outlined the technical pitfalls and challenges regarding the different EV-isolation and characterization techniques (Ramirez et al. 2018). Further investigations are required to compare the yield of EVs by the different isolation methods.
Intriguingly, a recent study by Kojima et al. reported a technique to enhance exosome biogenesis (Kojima et al. 2018). In the study, EVs biogenesis was increased by incorporation of a plasmid construct built to include EVs production booster genes. The team screened and identified STEAP3 (involved in exosome biogenesis), syndecan-4 (SDC4; supports budding of endosomal membranes to form multivesicular bodies), and a fragment of L-aspartate oxidase (NadB; possibly boosts cellular metabolism by tuning up the citric acid cycle) as potential synthetic exosome production boosters. They then incorporated these genes into tricistronic plasmid vectors and transfected into exosome-producer cells, which resulted in enhanced exosome production. Such techniques can be further studied to establish generalizability of the method to enhance EVs production.
Heterogeneity of EVs
As described previously, the composition and number of EVs released are fairly depend on the parent cell type and microenvironment (Takuma Yamashita et al. 2018) highlighting that selection of donor cells and culture condition to yield the EVs are crucial considerations in the clinical application of EVs. In vitro studies have shown that a large number of cells need to be cultured to yield a few micrograms of EVs (Lv et al. 2018). Several methods of enhancing EVs production have been suggested, such as increased intracellular calcium levels, thermal stimulation, hypoxia, changing the pH of the microenvironment, and use of chemotherapeutic agents (Harmati et al. 2017; Lv et al. 2018). However, investigations need to be performed to establish whether use of stressors also increases the release of contaminants in EVs and therefore might lead to adverse effects.
EVs collected from biofluids (blood, urine, breast milk, etc.) of interest, through different isolation techniques, may vary in quality. The purity and content of EVs many vary due to factors such as age, gender, ethnicity, body mass index, disease, use of medications, general lifestyle, and dietary habits. Apart from isolation techniques, the volume of biofluids, biofluid collection techniques, and storage conditions can all affect the biochemical properties and stability of EVs (Ramirez et al. 2018).
Loading of EVs
Lipid or polymer assisted delivery vehicles to allow large-scale production and flexible loading strategies (DeMarino et al. 2017). On the other hand, generation of EVs in mass quantities and subsequent loading for use in clinical purposes poses a challenge in the field (Sutaria et al. 2017; Takuma Yamashita et al. 2018). EVs biogenesis is an endogenous process. This allows for two broad strategies to load the EVs – (i) pre-isolation method, where parental cells are pre-treated with agents of interest followed by conditioning of the medium with drug-loaded EVs, which are then isolated, and (ii) post-isolation method, where agents of interest are actively or passively loaded after isolation of EVs (Rufino-Ramos et al. 2017).
Post-isolation methods include electroporation, saponin permeabilization, hypotonic dialysis, passive incubation, freeze-thaw cycle, sonication, and extrusion, with varying degree of advantages and disadvantages (Fuhrmann et al. 2015b; Haney et al. 2015; Sutaria et al. 2017). For instance, the electroporation technique, even though it is reported to not affect the EV-surface proteins, can cause EV-aggregation and changes in morphological characteristics (Sutaria et al. 2017). Saponin treatment to permeabilize EVs to load therapeutic cargoes has been compared to other methods, such as electroporation, sonication, freeze-thaw, and direct incubation (Sutaria et al. 2017). Saponin treatment did not affect EV-size distribution, surface charge, or morphology (Fuhrmann et al. 2015a, 2015b; Sutaria et al. 2017). Even though saponin treatment has been reported to be an easy method of EVs loading (Sutaria et al. 2017), further investigations are required to test if this method affects the integrity of EV-membrane, thereby immunogenicity. Sonication was shown to result in more efficient loading than saponin permeabilization and lower therapeutic efficiency upon intranasal administration of catalase loaded EVs (Haney et al. 2015). Such lower therapeutic efficiency can be explained by the disruption of EV-membrane integrity, which exposes the cargo to reticuloendothelial system-based degradation. Regardless of the method applied, EVs number and cargo concentration need to be properly optimized to overcome the challenges in clinical applications of EV-based therapeutics. Overcoming such challenges will allow efficient loading strategies to produce a detectable therapeutic response and improved pharmacokinetics of cargo loaded EVs.
Interestingly, to circumvent the EV-loading issues, Kojima et al. developed an EXOsomal transfer into cells (EXOtic) devices enabling efficient, customizable production of designer exosomes. The team used exosome producer cells with EXOtic devices that increased exosome production, enabled specific mRNA packaging, and delivery of the therapeutic mRNA into the cytosol of target cells in vitro and in vivo. They used an in vivo PD model to implant the engineered mammalian cells with EXOtic devices to show that catalase mRNA containing designer exosomes can be delivered to the target cells. Their results showed the attenuation of neurotoxicity and neuroinflammation associated with Parkinson’s pathology, indicating the potential therapeutic applications of designer exosomes in RNA delivery (Kojima et al. 2018).
Targeting of EVs
Once delivering the therapeutic agents to the target cells by EVs, the EVs interact with the target cells in different ways (Alvarez-Erviti et al. 2011; Haney et al. 2015). EVs fuse with target cell membranes, either directly with the plasma membrane or with the endosomal membrane after endocytic uptake (Rooj et al. 2016). Cells appear to take up EVs by a variety of endocytic pathways, including clathrin-dependent endocytosis, and clathrin-independent pathways such as caveolin-mediated uptake, macropinocytosis, phagocytosis, and lipid raft-mediated internalization (Costa Verdera et al. 2017; Mulcahy et al. 2014). As EVs consist of a heterogeneous population of vesicles, it is likely that they enter cells via multiple mechanisms that may depend on EV-surface or target cell surface composition. The underlying mechanism of EV-target cell interactions to allow effective drug delivery is poorly understood (Mulcahy et al. 2014). On the other hand, interactions of designed lipid (Braun et al. 2016) or polymeric systems (Ahn et al. 2013) are well understood. Smart designing strategies that exist for such exogenous carriers can be applied to the EVs to see if they work for such endogenous vehicles.
Biodistribution of EVs
Understanding the in vivo fate of EVs is another aspect of EV biology that requires extensive evaluation prior to considering the feasibility of employing EVs as drug delivery vehicles. A biodistribution investigation in vivo compared the three different mouse cell sources, along with different routes of administration and doses of EVs, all of which showed to affect the biodistribution pattern (Wiklander et al. 2015). To understand the spatiotemporal distribution of EVs in vivo i.e., tissue distribution, blood levels, and urine clearance, an effective real-time EV-tracking system is required. So far, the use of bioluminescence EVs labelling with multimodal EVs imaging reporter Gaussia luciferase conjugated to biotin acceptor protein and the transmembrane domain of platelet-derived growth factor receptor (GlucB) (Lai et al. 2014) and Renilla luciferase (Rluc) (Gangadaran et al. 2017) are serving as powerful reporters of in vivo EVs distribution. Also, fluorescence EV-labelling where EV proteins are fused with recombinant proteins (GFP or RFP) or organic fluorescent dyes (such as lipophilic DiR, PKH67, or PHH26) are also allowing successful in vivo imaging. Although the bioluminescence imaging (BLI) and fluorescence imaging (FLI) allow tracking of EVs with in vivo imaging system (IVIS), further investigations are required to address the drawbacks of the individual systems. Such drawbacks include toxicity and half-life of bioluminescence reporters, efficiency of EV labelling, dependence of florescence intensity on protein expression, effect of fluorescent dyes on EV cargo content due to steric hindrance, false positive signals due to aggregation of dyes, and persistence of fluorescent dyes in systemic circulation outlasting EVs (Chuo et al. 2018). Currently, single photon emission computed tomography (SPECT) and positron emission tomography (PET) imaging systems for radiolabeled EVs, and MRI imaging system for ultrasmall superparamagnetic iron oxide particle (USPIO)-loaded EVs are employed to monitor distribution of EVs (Chuo et al. 2018). Recently, a novel exosome labelling technique was described by Betzer et al. where MSC-derived exosomes with glucose-coated gold nanoparticle (GNP) labeling were used to track EVs by computed tomography (CT) (Betzer et al. 2018). Overall, establishing effective means of in vivo EV-tracking will allow better design and delivery investigations of EVs for therapeutic applications.
Potential for EVs in Management of NeuroAIDS
EVs and HIV
EVs have been implicated in HIV infection and dissemination (Schorey et al. 2015). Many HIV components have been characterized in the cargo of EVs, as listed in Table 4. Consequences of exosomal delivery of HIV-elements include reactivation of viral replication in latent cells (Tang et al. 2018), inflammation (Bernard et al. 2014; Sampey et al. 2016), immunodeficiency (Lenassi et al. 2010), and possibly the BBB leakiness which allow neuro-invasion (Atluri et al. 2015; Raymond et al. 2016). In the clinical context, the presence of HIV-elements as exosomal cargo can contribute to neuroinflammation, neurodegeneration, and HIV-associated neurocognitive disorders (HAND). The distinct effects of EVs, such as promoting or halting pathogenesis, appears to be dependent on the source and composition of EVs (Teow et al. 2016). Apart from reports of EV mediated HIV pathogenesis, there are a few reports claiming that EVs may potentially inhibit HIV infection (Rufino-Ramos et al. 2017; Teow et al. 2016; Tumne et al. 2009). T-cells, despite being the primary reservoirs of HIV infection, releases exosomes with inhibitory effects against HIV, reported to act via HIV -1 transcription inhibition (Tumne et al. 2009). CD4+- containing exosomes from T-cells have been reported to inhibit HIV infection as opposed to the negative regulatory factor (Nef)-induced CD4-depleted exosomal activity, potentially via masking of HIV envelope protein needed for spreading the virus (de Carvalho et al. 2014). Interleukins, interferon-alpha, interferon beta, and tumor necrosis factor are other exosomal molecules that have previously been reported to contribute to inhibition of HIV infection (Teow et al. 2016). Another recent study suggests the potential role of astroglial exosomes against smoking-induced oxidative stress and HIV-1 replication in the CNS (Ranjit et al. 2018). Considering that EVs have some role to play in the inhibition of HIV infection, extensive studies to explore the biodistribution, BBB penetration, and potential use of such anti-HIV EVs as delivery vehicles can be formulated.
EVs are produced by almost all kinds of cells, and there are many similarities in the biogenesis of EVs and HIV as often HIV hijacks EV biogenesis machinery to release its virions into extracellular space (Patters and Kumar 2018). The resemblance of EVs and HIV is not limited to size, structure, cargo but also is in their mode of entry (via fusion or endocytosis) to the target cells (Nolte-'t Hoen et al. 2016). Therefore, EVs shedding from infected cells can incorporate viral elements inside them and thus sometimes referred to as non-infectious viruses. Thereby EVs isolated from HIV-infected cells would be contaminated with HIV particles (Nolte-'t Hoen et al. 2016).
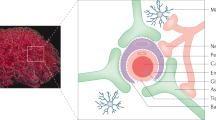
Morphologically, EVs and HIV are both lipid membrane-bound particles of 100–200 nm in diameter as shown in Fig. 1 and their lipid membrane presents significantly higher levels of cholesterol and glycosphingolipids as compared to cell membranes (Izquierdo-Useros et al. 2011). As EVs and HIV virions utilize similar pathways, there are similarities in their membrane protein content (enriched in tetraspanins and MHC-related proteins) and cytoplasmic proteins content (actin, TSG101, heat shock protein) (Izquierdo-Useros et al. 2011). Also, identical host cell protein profiles were found upon analyses of HIV-1 particles and exosomes from macrophages (Nguyen et al. 2003). Additionally, EVs characterized from HIV infected materials contain viral components including, RNA, envelope, Nef, Gag, and transactivating response (TAR) (Chahar et al. 2015). These morphological and biochemical similarities, together with the incorporation of host components, create an obstacle for pure EVs isolation (Izquierdo-Useros et al. 2011). Thus, more in-depth knowledge is required to understand the extent of mimicry between EVs and HIV-1 to be able to develop isolation strategies and treatment of HAND using EVs as a drug delivery vehicle.
Challenges of EVs Separation from HIV
Owing to the similar size (HIV ranges from 100 to 120 nm compared to 30–150 nm diameter of EVs), as shown in Fig. 1, and buoyant characteristics, conventional method of isolating EVs based on buoyant density (EVs: 1.13–1.18 g/L; most retroviruses: 1.16–1.18 g/L) proved ineffective (Cantin et al. 2008). Using immunoaffinity capture techniques against distinctive EV markers such as CD81, CD63, and CD45, EVs can be separated from HIV; however, the immunogenic capabilities of an EV might be compromised due to the possibility of not representing the specific marker on its surface within a given class of EVs (Witwer et al. 2013). Such dependence on physical properties for separation may lead to lower yields of EVs. To overcome these limitations, Konadu et al. applied iodixanol velocity gradients, an advanced density gradient technique to separate EVs from HIV particles (Konadu et al. 2016). They observed that the EVs containing fraction separated at lower density while HIV particles segregated at high-density fractions. Work by Hoen et al. showed success in both quantitative and qualitative flow cytometric separation strategy that is based on antigenic presentations on EVs (Hoen et al. 2012). Research works from Dr. Kashanchi’s laboratory have demonstrated the ability of nanotrap particles to capture HIV molecules, NT082 and NT084 particles for Tat protein, NT080 for Nef protein, NT073, and NT086 for HIV particles (Jaworski et al. 2014). Nanotrap particles are hydrogel particles with a core consisting of cross-linked polymeric networks of N-isopropylacrylamide (NIPAm) and co-monomers such as acrylic acid (AAc), allylamine (AA), and N,N′-methylenebisacrylamide (BIS) (Shafagati et al. 2015). The outer shell consists of inert cross-linked poly(p)-NIPAm shell or contains vinyl sulfonic acid (VSA). These particles, sizes ranging from 300 nm to 3000 nm, act as affinity baits to capture the target proteins. Such nanotrap technology may be used to concentrate HIV proteins and virions from infected samples to increase HIV detection. Further investigations in this field are needed to develop effective alternative strategies for EVs and HIV separation.
Disruption of the BBB in HIV infected individuals were reported to occur due to viral proteins such as glycoprotein (gp120), Nef, and transactivator of transcription (Tat) (Atluri et al. 2015; Kanmogne et al. 2007). Low penetration of ARV drugs to the CNS, in addition to the fact that the CNS is a significant viral reservoir, paves the way for development of HAND. This condition can be improved using ARV drugs; however, the challenge faced is the efficiency of drug penetration into the CNS. Therefore, novel strategies are needed to improve penetration of ARV drugs across the BBB to successfully cure HAND (DeMarino et al. 2017). Recently, research has intensified to develop and use carrier systems, such as nanocarriers with surface alterations, to target drugs to CNS and improve drug bioavailability (Kuo and Chen 2006; Wong et al. 2010).
Existing Nanotechnology in Management of NeuroAIDS
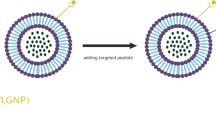
Many preclinical nanomaterial-based CNS delivery of ARV drugs for the prevention or treatment of HIV have been reported. Use of polymeric delivery systems has been studied by Kuo et al. (Kuo and Su 2007) comparing drug loading efficiencies and improved BBB drug trafficking of drugs such as stavudine, delavirdine, and saquinavir. The study also investigated the efficiency of drug delivery by lipid-based nanocarriers, such as solid-lipid nanoparticles, that showed improved CNS bioavailability. Use of siRNA that target HIV genes like Nef, viral protein X (Vpr), Tat, Gag, DNA polymerase (Pol), Env, viral infectivity factor (Vif), transactivating regulatory protein for viral protein expression (Rev), and transactivation response element (TAR) have also been explored as prospective tools for antiviral therapy (Hu et al. 2016; Levanova and Poranen 2018). Cellular proteins such as CCR5 and CXCR4, co-receptors for viral entry, have also been considered as siRNA targets for vaccination purposes (DeMarino et al. 2017). Liposomes and polymeric nanoparticles were investigated to carry anti-CCR5 siRNA to reduce CCR5 expression (DeMarino et al. 2017). More recently, Roy et al., (Roy et al. 2018) performed in vitro characterization of nanodiamonds to carry the ARV drug efavirenz. Nanodiamonds have an assortment of functional groups, most of which are oxygenated moieties, including carboxylic acid, lactone, ketone, ether, hydroxyl, etc., on their surface and are biocompatible and structurally stable with low cytotoxicity. Nanodiamonds were shown to be capable of encapsulating hydrophobic ARV drugs, thereby improving drug stability and half-life. Another study by Kaushik et al. explored the use of magnetically-guided delivery of 3′-Azido-2′,3′-dideoxythymidine-5′-triphosphate (an antiviral nucleotide) to traffic drugs across the BBB (Kaushik et al. 2016), as shown in Fig. 2. Such magneto-electric nanocarriers have shown potential in delivery of agents such as brain-derived neurotrophic factor (BDNF), TIMP-1, and Beclin1siRNA in in vitro models, urging further testing in animal models (Kaushik et al. 2018).
Various Extracellular Vesicle, Liposome, and Magnetic Nanoparticle, utilized for delivery of active molecules to BBB. a; A representative schematic of EV used for delivering RNAs, anti-RNAs, proteins, small molecules and imaging molecules. b; Liposome nanocarrier schematic with aqueous core containing hydrophilic molecules. The aqueous core surrounded by lipid bilayer encapsulate hydrophobic agents. C; Schematics of magnetic nanoparticles. Magnetic nanoparticles consist of a magnetic core surrounded by lipid layer encapsulating drugs. PEGlyated lipid layer have been to be effective in decorating RGD on the surface of magnetic nanoparticle
EVs were shown to transport molecules between cells, including HIV derived proteins and RNA, for pathogenic effects, as shown by Sampey et al. (Sampey et al. 2016). This study showed that exosomes from uninfected cells could activate latent HIV in infected cells in vitro, possibly via increased RNA Polymerase II loading onto the HIV promoter in the infected cells (Barclay et al. 2017). Such reactivation of latent HIV viruses may make them accessible to ARV drugs, opening the possibility of using such exosomes as adjunctive treatment in combination with existing ARV treatment modalities. Owing the ability of EVs to deliver cargoes across the BBB, strategies to use EVs as therapeutic conduits to deliver drugs to the brain are under investigation. Extensive investigations on EV-mediated CNS drug delivery of ARV drugs are warranted in the field.
Conclusions
It is now well established that EVs, the nanovesicles, play a role in intercellular communication under normal physiology as well as pathological conditions, such as HIV infection (Patters and Kumar 2018). Researchers are faced with many challenges when experimenting with EVs and HIV. Complications arise when experiments require EVs and HIV separation, owing to their common structural and molecular properties (Nolte-'t Hoen et al. 2016). Elucidating the pathogenic mechanisms of viral protein-mediated neurotoxicity that involves EVs is an actively researched area (Crews et al. 2009; Patters and Kumar 2018). Also, development of effective pharmacological amelioration strategies to overcome the detrimental effects of HIV and eradication of HIV reservoirs are in dire need, especially in people with HAND. With the advent of nanotechnology, varieties of nanocarriers are explored as an option for delivering drug(s) to the CNS. EVs, particularly exosomes, show promise as drug delivery vehicles. The inherent capacity of exosomes to enclose biological and chemical agents, ease of surface modification and their endogenous nature confers advantage of EVs as potential drug delivery vehicles. However, further investigations are required to overcome the challenges faced in the EVs research field prior to considering EVs as potential drug delivery systems. Future investigations can focus on the challenges faced in the EVs research field such as those concerned with improved isolation, loading, characterization, quantification, and real-time in vivo tracking strategies of EVs.
References
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37(1):13–25. https://doi.org/10.1016/j.nbd.2009.07.030
Abels ER, Breakefield XO (2016) Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 36(3):301–312. https://doi.org/10.1007/s10571-016-0366-z
Ahn S, Seo E, Kim K, Lee SJ (2013) Controlled cellular uptake and drug efficacy of nanotherapeutics. Sci Rep 3:1997. https://doi.org/10.1038/srep01997
Almutairi MM, Gong C, Xu YG, Chang Y, Shi H (2016) Factors controlling permeability of the blood-brain barrier. Cell Mol Life Sci 73(1):57–77. https://doi.org/10.1007/s00018-015-2050-8
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345. https://doi.org/10.1038/nbt.1807
Antimisiaris SG, Mourtas S, Marazioti A (2018) Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics 10(4):218. https://doi.org/10.3390/pharmaceutics10040218
Antonyak MA, Cerione RA (2015) Emerging picture of the distinct traits and functions of microvesicles and exosomes. Proc Natl Acad Sci U S A 112(12):3589–3590. https://doi.org/10.1073/pnas.1502590112
Arvanitis CD, McDannold N (2015) Chapter 18 - drug delivery to the brain via focused ultrasound. In: Golby AJ (ed) Image-Guided Neurosurgery. Academic Press, Boston, pp 441–474
Atluri VS, Hidalgo M, Samikkannu T, Kurapati KR, Jayant RD, Sagar V, Nair MP (2015) Effect of human immunodeficiency virus on blood-brain barrier integrity and function: an update. Front Cell Neurosci 9:212. https://doi.org/10.3389/fncel.2015.00212
Barclay RA, Schwab A, DeMarino C, Akpamagbo Y, Lepene B, Kassaye S, Iordanskiy S, Kashanchi F (2017) Exosomes from uninfected cells activate transcription of latent HIV-1. J Biol Chem 292(36):14764. https://doi.org/10.1074/jbc.A117.793521
Bernard MA, Zhao H, Yue SC, Anandaiah A, Koziel H, Tachado SD (2014) Novel HIV-1 MiRNAs stimulate TNFα release in human macrophages via TLR8 signaling pathway. PLoS One 9(9):e106006. https://doi.org/10.1371/journal.pone.0106006
Betzer, O., Perets, N., Barnoy, E., Offen, D., & Popovtzer, R. (2018). Labeling and tracking exosomes within the brain using gold nanoparticles (Vol. 10506): SPIE
Braun T, Kleusch C, Naumovska E, Merkel R, Csiszar A (2016) A bioanalytical assay to distinguish cellular uptake routes for liposomes. Cytometry A 89(3):301–308. https://doi.org/10.1002/cyto.a.22792
Cantin R, Diou J, Belanger D, Tremblay AM, Gilbert C (2008) Discrimination between exosomes and HIV-1: purification of both vesicles from cell-free supernatants. J Immunol Methods 338(1–2):21–30. https://doi.org/10.1016/j.jim.2008.07.007
Chahar HS, Bao X, Casola A (2015) Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses 7(6):3204–3225. https://doi.org/10.3390/v7062770
Chen CC, Liu L, Ma F, Wong CW, Guo XE, Chacko JV et al (2016) Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell Mol Bioeng 9(4):509–529. https://doi.org/10.1007/s12195-016-0458-3
Chuo ST-Y, Chien JC-Y, Lai CP-K (2018) Imaging extracellular vesicles: current and emerging methods. J Biomed Sci 25(1):91–91. https://doi.org/10.1186/s12929-018-0494-5
Cooper JM, Wiklander PB, Nordin JZ, Al-Shawi R, Wood MJ, Vithlani M et al (2014) Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord 29(12):1476–1485. https://doi.org/10.1002/mds.25978
Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P (2017) Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J Control Release 266:100–108. https://doi.org/10.1016/j.jconrel.2017.09.019
Crews L, Patrick C, Achim CL, Everall IP, Masliah E (2009) Molecular pathology of neuro-AIDS (CNS-HIV). Int J Mol Sci 10(3):1045–1063. https://doi.org/10.3390/ijms10031045
Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G (2008) Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal Cancer. Mol Ther 16(4):782–790. https://doi.org/10.1038/mt.2008.1
de Carvalho JV, de Castro RO, da Silva EZ, Silveira PP, da Silva-Januario ME, Arruda E et al (2014) Nef neutralizes the ability of exosomes from CD4+ T cells to act as decoys during HIV-1 infection. PLoS One 9(11):e113691. https://doi.org/10.1371/journal.pone.0113691
DeMarino C, Schwab A, Pleet M, Mathiesen A, Friedman J, El-Hage N, Kashanchi F (2017) Biodegradable nanoparticles for delivery of therapeutics in CNS infection. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 12(1):31–50. https://doi.org/10.1007/s11481-016-9692-7
Didiot MC, Hall LM, Coles AH, Haraszti RA, Godinho BM, Chase K et al (2016) Exosome-mediated delivery of Hydrophobically modified siRNA for huntingtin mRNA silencing. Mol Ther 24(10):1836–1847. https://doi.org/10.1038/mt.2016.126
Doeppner TR, Bahr M, Giebel B, Hermann DM (2018) Immunological and non-immunological effects of stem cell-derived extracellular vesicles on the ischaemic brain. Ther Adv Neurol Disord 11:1756286418789326. https://doi.org/10.1177/1756286418789326
Druzhkova TA, Yakovlev AA (2018) Exosome drug delivery through the blood–brain barrier: experimental approaches and potential applications. Neurochem J 12(3):195–204. https://doi.org/10.1134/S1819712418030030
Duechler M (2013) Vehicles for small interfering RNA transfection: exosomes versus synthetic Nanocarriers. DNA and RNA Nanotechnology 1
Duncan R, Gaspar R (2011) Nanomedicine(s) under the microscope. Mol Pharm 8(6):2101–2141. https://doi.org/10.1021/mp200394t
Fu H, McCarty DM (2016) Crossing the blood–brain-barrier with viral vectors. Curr Opin Virol 21:87–92. https://doi.org/10.1016/j.coviro.2016.08.006
Fuhrmann G, Herrmann IK, Stevens MM (2015a) Cell-derived vesicles for drug therapy and diagnostics: opportunities and challenges. Nano Today 10(3):397–409. https://doi.org/10.1016/j.nantod.2015.04.004
Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM (2015b) Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release 205:35–44. https://doi.org/10.1016/j.jconrel.2014.11.029
Gabathuler R (2010) Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol Dis 37(1):48–57. https://doi.org/10.1016/j.nbd.2009.07.028
Gangadaran, P., Li, X. J., Lee, H. W., Oh, J. M., Kalimuthu, S., Rajendran, R. L., ... Ahn, B. C. (2017). A new bioluminescent reporter system to study the biodistribution of systematically injected tumor-derived bioluminescent extracellular vesicles in mice. Oncotarget, 8(66), 109894-109914. Doi: https://doi.org/10.18632/oncotarget.22493
Gilligan KE, Dwyer RM (2017) Engineering exosomes for Cancer therapy. Int J Mol Sci 18(6). https://doi.org/10.3390/ijms18061122
Gourlay J, Morokoff AP, Luwor RB, Zhu HJ, Kaye AH, Stylli SS (2017) The emergent role of exosomes in glioma. J Clin Neurosci 35:13–23. https://doi.org/10.1016/j.jocn.2016.09.021
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z et al (2015) Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release 207:18–30. https://doi.org/10.1016/j.jconrel.2015.03.033
Harmati M, Tarnai Z, Decsi G, Kormondi S, Szegletes Z, Janovak L et al (2017) Stressors alter intercellular communication and exosome profile of nasopharyngeal carcinoma cells. J Oral Pathol Med 46(4):259–266. https://doi.org/10.1111/jop.12486
Heinemann ML, Ilmer M, Silva LP, Hawke DH, Recio A, Vorontsova MA et al (2014) Benchtop isolation and characterization of functional exosomes by sequential filtration. J Chromatogr A 1371:125–135. https://doi.org/10.1016/j.chroma.2014.10.026
Hersh DS, Wadajkar AS, Roberts N, Perez JG, Connolly NP, Frenkel V et al (2016) Evolving drug delivery strategies to overcome the blood brain barrier. Curr Pharm Des 22(9):1177–1193
Hoen ENMNT, van der Vlist EJ, Aalberts M, Mertens HCH, Bosch BJ, Bartelink W et al (2012) Quantitative and qualitative flow cytometric analysis of nanosized cell-derived membrane vesicles. Nanomedicine 8(5):712–720. https://doi.org/10.1016/j.nano.2011.09.006
Hu G, Yang L, Cai Y, Niu F, Mezzacappa F, Callen S et al (2016) Emerging roles of extracellular vesicles in neurodegenerative disorders: focus on HIV-associated neurological complications. Cell Death Dis 7(11):e2481–e2481. https://doi.org/10.1038/cddis.2016.336
Huey R, Hawthorne S, McCarron P (2017) The potential use of rabies virus glycoprotein-derived peptides to facilitate drug delivery into the central nervous system: a mini review. J Drug Target 25(5):379–385. https://doi.org/10.1080/1061186x.2016.1223676
Izquierdo-Useros N, Puertas MC, Borras FE, Blanco J, Martinez-Picado J (2011) Exosomes and retroviruses: the chicken or the egg? Cell Microbiol 13(1):10–17. https://doi.org/10.1111/j.1462-5822.2010.01542.x
Jan AT, Malik MA, Rahman S, Yeo HR, Lee EJ, Abdullah TS, Choi I (2017) Perspective insights of exosomes in neurodegenerative diseases: a critical appraisal. Front Aging Neurosci 9:317–317. https://doi.org/10.3389/fnagi.2017.00317
Jawahar N, Meyyanathan S (2012) Polymeric nanoparticles for drug delivery and targeting: a comprehensive review. Review Article 1(4):217–223. https://doi.org/10.4103/2278-344x.107832
Jaworski E, Saifuddin M, Sampey G, Shafagati N, Van Duyne R, Iordanskiy S et al (2014) The use of Nanotrap particles Technology in Capturing HIV-1 Virions and viral proteins from infected cells. PLoS One 9(5):e96778. https://doi.org/10.1371/journal.pone.0096778
Johnston MJ, Semple SC, Klimuk SK, Ansell S, Maurer N, Cullis PR (2007) Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim Biophys Acta 1768(5):1121–1127. https://doi.org/10.1016/j.bbamem.2007.01.019
Kang YJ, Cutler EG, Cho H (2018) Therapeutic nanoplatforms and delivery strategies for neurological disorders. Nano Convergence 5(1):35–35. https://doi.org/10.1186/s40580-018-0168-8
Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y (2007) HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. J Cereb Blood Flow Metab 27(1):123–134. https://doi.org/10.1038/sj.jcbfm.9600330
Katakowski M, Buller B, Zheng X, Lu Y, Rogers T, Osobamiro O et al (2013) Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett 335(1):201–204. https://doi.org/10.1016/j.canlet.2013.02.019
Katsiougiannis S (2015) Extracellular Vesicles: Evolving Contributors in Autoimmunity. ForumImmunDisTher, 6(3-4), 163-170. https://doi.org/10.1615/ForumImmunDisTher.2016016491
Kaushik A, Jayant RD, Nikkhah-Moshaie R, Bhardwaj V, Roy U, Huang Z et al (2016) Magnetically guided central nervous system delivery and toxicity evaluation of magneto-electric nanocarriers. Sci Rep 6:25309. https://doi.org/10.1038/srep25309
Kaushik A, Jayant RD, Nair M (2018) Nanomedicine for neuroHIV/AIDS management. Nanomedicine (Lond) 13(7):669–673. https://doi.org/10.2217/nnm-2018-0005
Kevadiya BD, Woldstad C, Ottemann BM, Dash P, Sajja BR, Lamberty B et al (2018) Multimodal Theranostic Nanoformulations permit magnetic resonance bioimaging of antiretroviral drug particle tissue-cell biodistribution. Theranostics 8(1):256–276. https://doi.org/10.7150/thno.22764
Kodidela S, Wang Y, Patters BJ, Gong Y, Sinha N, Ranjit S et al (2019) Proteomic profiling of exosomes derived from plasma of HIV-infected alcohol drinkers and cigarette smokers. J NeuroImmune Pharmacol. https://doi.org/10.1007/s11481-019-09853-2
Kojima R, Bojar D, Rizzi G, Hamri GC-E, El-Baba MD, Saxena P et al (2018) Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun 9(1):1305. https://doi.org/10.1038/s41467-018-03733-8
Konadu KA, Huang MB, Roth W, Armstrong W, Powell M, Villinger F, Bond V (2016) Isolation of exosomes from the plasma of HIV-1 positive individuals. J Vis Exp 107. https://doi.org/10.3791/53495
Kuo YC, Chen HH (2006) Effect of nanoparticulate polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate on the permeability of zidovudine and lamivudine across the in vitro blood-brain barrier. Int J Pharm 327(1–2):160–169. https://doi.org/10.1016/j.ijpharm.2006.07.044
Kuo YC, Su FL (2007) Transport of stavudine, delavirdine, and saquinavir across the blood-brain barrier by polybutylcyanoacrylate, methylmethacrylate-sulfopropylmethacrylate, and solid lipid nanoparticles. Int J Pharm 340(1–2):143–152. https://doi.org/10.1016/j.ijpharm.2007.03.012
Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW et al (2014) Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8(1):483–494. https://doi.org/10.1021/nn404945r
Lanone S, Boczkowski J (2006) Biomedical applications and potential health risks of nanomaterials: molecular mechanisms. Curr Mol Med 6(6):651–663
Lee K, Shao H, Weissleder R, Lee H (2015) Acoustic purification of extracellular microvesicles. ACS Nano 9(3):2321–2327. https://doi.org/10.1021/nn506538f
Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y et al (2010) HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 11(1):110–122. https://doi.org/10.1111/j.1600-0854.2009.01006.x
Levanova A, Poranen MM (2018) RNA interference as a prospective tool for the control of human viral infections. Front Microbiol 9:2151–2151. https://doi.org/10.3389/fmicb.2018.02151
Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X et al (2015) Targeted exosome-mediated delivery of opioid receptor mu siRNA for the treatment of morphine relapse. Sci Rep 5:17543. https://doi.org/10.1038/srep17543
Liu C, Guo J, Tian F, Yang N, Yan F, Ding Y et al (2017) Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano 11(7):6968–6976. https://doi.org/10.1021/acsnano.7b02277
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D (2017) Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin 38(6):754–763. https://doi.org/10.1038/aps.2017.12
Lv L-L, Wu W-J, Feng Y, Li Z-L, Tang T-T, Liu B-C (2018) Therapeutic application of extracellular vesicles in kidney disease: promises and challenges. J Cell Mol Med 22(2):728–737. https://doi.org/10.1111/jcmm.13407
Madison MN, Okeoma CM (2015) Exosomes: implications in HIV-1 pathogenesis. Viruses 7(7):4093–4118. https://doi.org/10.3390/v7072810
Mäger I, Meyer AH, Li J, Lenter M, Hildebrandt T, Leparc G, Wood MJA (2017) Targeting blood-brain-barrier transcytosis – perspectives for drug delivery. Neuropharmacology 120:4–7. https://doi.org/10.1016/j.neuropharm.2016.08.025
Marianecci C, Rinaldi F, Hanieh PN, Di Marzio L, Paolino D, Carafa M (2017) Drug delivery in overcoming the blood-brain barrier: role of nasal mucosal grafting. Drug Des Devel Ther 11:325–335. https://doi.org/10.2147/DDDT.S100075
Mathieu M, Martin-Jaular L, Lavieu G, Thery C (2019) Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21(1):9–17. https://doi.org/10.1038/s41556-018-0250-9
Matsumoto J, Stewart T, Banks WA, Zhang J (2017) The transport mechanism of extracellular vesicles at the blood-brain barrier. Curr Pharm Des 23(40):6206–6214. https://doi.org/10.2174/1381612823666170913164738
Mishra DK, Shandilya R, Mishra PK (2018) Lipid based nanocarriers: a translational perspective. Nanomedicine 14(7):2023–2050. https://doi.org/10.1016/j.nano.2018.05.021
Moghimi SM, Hunter AC, Murray JC (2005) Nanomedicine: current status and future prospects. FASEB J 19(3):311–330. https://doi.org/10.1096/fj.04-2747rev
Mulcahy, LA, Pink, RC, & Carter, DRF (2014). Routes and mechanisms of extracellular vesicle uptake. JEV, 3, 10.3402/jev.v3403.24641. https://doi.org/10.3402/jev.v3.24641
Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P (2013) Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma Multiforme cells conferred Chemosensitivity. Mol Ther Nucleic Acids 2(10):e126–e126. https://doi.org/10.1038/mtna.2013.60
Murphy, AM, & Rabkin, SD (2013). Current status of gene therapy for brain tumors. Transl Res. 161(4), 339-354. https://doi.org/10.1016/j.trsl.2012.11.003
Nair LS, Laurencin CT (2006) Polymers as biomaterials for tissue engineering and controlled drug delivery. Adv Biochem Eng Biotechnol 102:47–90
Nair M, Jayant RD, Kaushik A, Sagar V (2016) Getting into the brain: potential of nanotechnology in the management of NeuroAIDS. Adv Drug Deliv Rev 103:202–217. https://doi.org/10.1016/j.addr.2016.02.008
Nawaz M, Shah N, Zanetti BR, Maugeri M, Silvestre RN, Fatima F et al (2018) Extracellular vesicles and matrix remodeling enzymes: the emerging roles in extracellular matrix remodeling, progression of diseases and tissue repair. Cells 7(10). https://doi.org/10.3390/cells7100167
Neuwelt EA, Frenkel EP, Rapoport S, Barnett P (1980) Effect of osmotic blood-brain barrier disruption on methotrexate pharmacokinetics in the dog. Neurosurgery 7(1):36–43
Neviani P, Wise PM, Murtadha M, Liu CW, Wu C-H, Jong AY et al (2018) Natural killer-derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res, canres.0779.2018. https://doi.org/10.1158/0008-5472.CAN-18-0779
Nguyen DG, Booth A, Gould SJ, Hildreth JE (2003) Evidence that HIV budding in primary macrophages occurs through the exosome release pathway. J Biol Chem 278(52):52347–52354. https://doi.org/10.1074/jbc.M309009200
Nolte-'t Hoen E, Cremer T, Gallo RC, Margolis LB (2016) Extracellular vesicles and viruses: are they close relatives? Proc Natl Acad Sci U S A 113(33):9155–9161. https://doi.org/10.1073/pnas.1605146113
Oksvold MP, Neurauter A, Pedersen KW (2015) Magnetic bead-based isolation of exosomes. Methods Mol Biol 1218:465–481. https://doi.org/10.1007/978-1-4939-1538-5_27
Oosthuyzen W, Sime NE, Ivy JR, Turtle EJ, Street JM, Pound J et al (2013) Quantification of human urinary exosomes by nanoparticle tracking analysis. J Physiol 591(23):5833–5842. https://doi.org/10.1113/jphysiol.2013.264069
Pacienza N, Lee RH, Bae EH, Kim DK, Liu Q, Prockop DJ, Yannarelli G (2019) In vitro macrophage assay predicts the in vivo anti-inflammatory potential of exosomes from human mesenchymal stromal cells. Mol Ther Methods Clin Dev 13:67–76. https://doi.org/10.1016/j.omtm.2018.12.003
Pardridge WM (2012) Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 32(11):1959–1972. https://doi.org/10.1038/jcbfm.2012.126
Patters BJ, Kumar S (2018) The role of exosomal transport of viral agents in persistent HIV pathogenesis. Retrovirology 15(1):79. https://doi.org/10.1186/s12977-018-0462-x
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D (2019) Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease. J Neuro-Oncol. https://doi.org/10.1007/s13365-018-0695-4
Quek C, Hill AF (2017) The role of extracellular vesicles in neurodegenerative diseases. Biochem Biophys Res Commun 483(4):1178–1186. https://doi.org/10.1016/j.bbrc.2016.09.090
Rajagopal C, Harikumar KB (2018) The origin and functions of exosomes in Cancer. Front Oncol 8:66–66. https://doi.org/10.3389/fonc.2018.00066
Ramirez MI, Amorim MG, Gadelha C, Milic I, Welsh JA, Freitas VM et al (2018) Technical challenges of working with extracellular vesicles. Nanoscale 10(3):881–906. https://doi.org/10.1039/c7nr08360b
Ranjit S, Patters BJ, Gerth KA, Haque S, Choudhary S, Kumar S (2018) Potential neuroprotective role of astroglial exosomes against smoking-induced oxidative stress and HIV-1 replication in the central nervous system. Expert Opin Ther Targets 22(8):703–714. https://doi.org/10.1080/14728222.2018.1501473
Raymond AD, Diaz P, Chevelon S, Agudelo M, Yndart-Arias A, Ding H et al (2016) Microglia-derived HIV Nef+ exosome impairment of the blood-brain barrier is treatable by nanomedicine-based delivery of Nef peptides. J Neuro-Oncol 22(2):129–139. https://doi.org/10.1007/s13365-015-0397-0
Rooj AK, Mineo M, Godlewski J (2016) MicroRNA and extracellular vesicles in glioblastoma: small but powerful. Brain tumor pathology 33(2):77–88. https://doi.org/10.1007/s10014-016-0259-3
Roy U, Drozd V, Durygin A, Rodriguez J, Barber P, Atluri V et al (2018) Characterization of Nanodiamond-based anti-HIV drug delivery to the brain. Sci Rep 8(1):1603. https://doi.org/10.1038/s41598-017-16703-9
Rufino-Ramos D, Albuquerque PR, Carmona V, Perfeito R, Nobre RJ, Pereira de Almeida L (2017) Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J Control Release 262:247–258. https://doi.org/10.1016/j.jconrel.2017.07.001
Sampey GC, Saifuddin M, Schwab A, Barclay R, Punya S, Chung M-C et al (2016) Exosomes from HIV-1-infected cells stimulate production of pro-inflammatory cytokines through trans-activating response (TAR) RNA. J Biol Chem 291(3):1251–1266. https://doi.org/10.1074/jbc.M115.662171
Schorey JS, Cheng Y, Singh PP, Smith VL (2015) Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep 16(1):24–43. https://doi.org/10.15252/embr.201439363
Shafagati N, Lundberg L, Baer A, Patanarut A, Fite K, Lepene B, Kehn-Hall K (2015) The use of Nanotrap particles in the enhanced detection of Rift Valley fever virus nucleoprotein. PLoS One 10(5):e0128215. https://doi.org/10.1371/journal.pone.0128215
Shah R, Patel T, Freedman JE (2018) Circulating extracellular vesicles in human disease. N Engl J Med 379(10):958–966. https://doi.org/10.1056/NEJMra1704286
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C et al (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18(9):1606–1614. https://doi.org/10.1038/mt.2010.105
Sutaria DS, Badawi M, Phelps MA, Schmittgen TD (2017) Achieving the promise of therapeutic extracellular vesicles: the devil is in details of therapeutic loading. Pharm Res 34(5):1053–1066. https://doi.org/10.1007/s11095-017-2123-5
Takeuchi H, Kojima H, Yamamoto H, Kawashima Y (2000) Polymer coating of liposomes with a modified polyvinyl alcohol and their systemic circulation and RES uptake in rats. J Control Release 68(2):195–205
Tan L, Wu H, Liu Y, Zhao M, Li D, Lu Q (2016) Recent advances of exosomes in immune modulation and autoimmune diseases. Autoimmunity 49(6):357–365. https://doi.org/10.1080/08916934.2016.1191477
Tang X, Lu H, Dooner M, Chapman S, Quesenberry PJ, Ramratnam B (2018) Exosomal tat protein activates latent HIV-1 in primary, resting CD4+ T lymphocytes. JCI Insight 3(7). https://doi.org/10.1172/jci.insight.95676
Teow SY, Nordin AC, Ali SA, Khoo AS (2016) Exosomes in human immunodeficiency virus type I pathogenesis: threat or opportunity? Adv Virol 2016:9852494–9852498. https://doi.org/10.1155/2016/9852494
Thakur A, Zou H, Yang M, Lee Y (2018) Abstract 3720: augmented loading efficiency of doxorubicin into glioma-derived exosomes by an integrated microfluidic device. Cancer Res 78(13 Supplement):3720. https://doi.org/10.1158/1538-7445.AM2018-3720
Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C et al (2018) Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 150:137–149. https://doi.org/10.1016/j.biomaterials.2017.10.012
Tumne, A., Prasad, V. S., Chen, Y., Stolz, D. B., Saha, K., Ratner, D. M., ... Gupta, P. (2009). Noncytotoxic suppression of human immunodeficiency virus type 1 transcription by exosomes secreted from CD8<sup>+</sup> T Cells. J Virol, 83(9), 4354. doi: https://doi.org/10.1128/JVI.02629-08
Turturici G, Tinnirello R, Sconzo G, Geraci F (2014) Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol Cell Physiol 306(7):C621–C633. https://doi.org/10.1152/ajpcell.00228.2013
Upadhyay RK (2014) Drug delivery systems, CNS protection, and the blood brain barrier. Biomed Res Int 2014:869269. https://doi.org/10.1155/2014/869269
van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML (2008) Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 47(5):1634–1643. https://doi.org/10.1002/hep.22236
van Tellingen O, Yetkin-Arik B, de Gooijer MC, Wesseling P, Wurdinger T, de Vries HE (2015) Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat 19:1–12. https://doi.org/10.1016/j.drup.2015.02.002
Viaud S, Ploix S, Lapierre V, Théry C, Commere P-H, Tramalloni D et al (2011) Updated technology to produce highly immunogenic dendritic cell-derived exosomes of clinical grade: a critical role of interferon-γ. J Immunother 34(1):65–75
Webber J, Clayton A (2013) How pure are your vesicles? JEV 2. https://doi.org/10.3402/jev.v2i0.19861
Welch JL, Stapleton JT, Okeoma CM (2019) Vehicles of intercellular communication: exosomes and HIV-1. https://doi.org/10.1099/jgv.0.001193
Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mager I et al (2015) Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4:26316. https://doi.org/10.3402/jev.v4.26316
Willis GR, Kourembanas S, Mitsialis SA (2017) Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front. Cardiovasc. Med. 4:63–63. https://doi.org/10.3389/fcvm.2017.00063
Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J et al (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2. https://doi.org/10.3402/jev.v2i0.20360
Wong HL, Chattopadhyay N, Wu XY, Bendayan R (2010) Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Adv Drug Deliv Rev 62(4–5):503–517. https://doi.org/10.1016/j.addr.2009.11.020
Wong HL, Wu XY, Bendayan R (2012) Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev 64(7):686–700. https://doi.org/10.1016/j.addr.2011.10.007
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M (2013a) Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 33(11):1711–1715. https://doi.org/10.1038/jcbfm.2013.152
Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y et al (2013b) MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells 31(12):2737–2746. https://doi.org/10.1002/stem.1409
Xin H, Katakowski M, Wang F, Qian J-Y, Liu XS, Ali MM et al (2017) MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 48(3):747–753. https://doi.org/10.1161/STROKEAHA.116.015204
Yamashita T, Takahashi Y, Nishikawa M, Takakura Y (2016) Effect of exosome isolation methods on physicochemical properties of exosomes and clearance of exosomes from the blood circulation. Eur J Pharm Biopharm 98:1–8. https://doi.org/10.1016/j.ejpb.2015.10.017
Yamashita T, Takahashi Y, Takakura Y (2018) Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull 41(6):835–842. https://doi.org/10.1248/bpb.b18-00133
Yáñez-Mó M, Siljander PRM, Andreu Z, Bedina Zavec A, Borràs FE, Buzas EI et al (2015) Biological properties of extracellular vesicles and their physiological functions. JEV 4(1):27066. https://doi.org/10.3402/jev.v4.27066
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R et al (2015) Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res 32(6):2003–2014. https://doi.org/10.1007/s11095-014-1593-y
You B, Xu W, Zhang B (2018) Engineering exosomes: a new direction for anticancer treatment. Am J Cancer Res 8(8):1332–1342
Yuan D, Zhao Y, Banks WA, Bullock KM, Haney M, Batrakova E, Kabanov AV (2017) Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 142:1–12. https://doi.org/10.1016/j.biomaterials.2017.07.011
Zagrean, A.-M., Hermann, D. M., Opris, I., Zagrean, L., & Popa-Wagner, A. (2018). Multicellular crosstalk between exosomes and the neurovascular unit after cerebral ischemia. Therapeutic Implications. Front. Neurosci, 12, 811–811. doi: https://doi.org/10.3389/fnins.2018.00811
Zarovni N, Corrado A, Guazzi P, Zocco D, Lari E, Radano G et al (2015) Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 87:46–58. https://doi.org/10.1016/j.ymeth.2015.05.028
Zeringer E, Barta T, Li M, Vlassov AV (2015) Strategies for isolation of exosomes. Cold Spring Harb Protoc 2015(4):319–323. https://doi.org/10.1101/pdb.top074476
Zhang Y, Hu YW, Zheng L, Wang Q (2017) Characteristics and roles of exosomes in cardiovascular disease. DNA Cell Biol 36(3):202–211. https://doi.org/10.1089/dna.2016.3496
Zhou Y, Peng Z, Seven ES, Leblanc RM (2018) Crossing the blood-brain barrier with nanoparticles. J. Control. Release 270:290–303. https://doi.org/10.1016/j.jconrel.2017.12.015
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC et al (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19(10):1769–1779. https://doi.org/10.1038/mt.2011.164
Funding
This study was funded by NIH/NIDA R01 grant awarded to SVY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author FS declares no conflict of interest. The author SC declares no conflict of interest and the author SVY declare no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahjin, F., Chand, S. & Yelamanchili, S.V. Extracellular Vesicles as Drug Delivery Vehicles to the Central Nervous System. J Neuroimmune Pharmacol 15, 443–458 (2020). https://doi.org/10.1007/s11481-019-09875-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-019-09875-w