Abstract
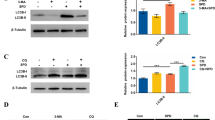
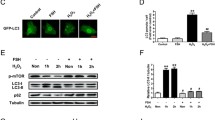
Sertoli cells (SCs) are necessary for proper germ cell development and viability. Unc-51 like autophagy activating kinase (ULK1) protein kinase is an important regulator of autophagy activation. This study aims to investigate the role of autophagy promoter ULK1 on cell viability of goat SCs. Our results showed that ULK1 knockdown in goat SCs decreased autophagy activation, which was confirmed by decreased expression of autophagy-related markers including LC3, Beclin1, Atg5, and Atg7 (P < 0.05). Meanwhile, lower ULK1 levels resulted in decreased expressions of goat SC marker genes ABP, AMH, FASL, and GATA4. However, a reverse trend of these parameters occurred when the goat SCs were transfected with ULK1 overexpression construct; higher ULK1 levels in goat SCs also decreased the ratio of Bax/Bcl-2. Moreover, ULK1 overexpression in goat SCs activated the autophagy levels when cells were exposed to an environmental contaminant bisphenol A (BPA). The above results indicated that ULK1 gene might play important roles in goat SC function by regulating cell viability.






Similar content being viewed by others
References
Alers S, Löffler AS, Wesselborg S, Stork B (2012) Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32:2–11
Antico Arciuch VG, Elguero ME, Poderoso JJ, Carreras MC (2012) Mitochondrial regulation of cell cycle and proliferation. Antioxid Redox Signal 16:1150–1180
Chen Y, Azad MB, Gibson SB (2009) Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ 16:1040–1052
Chen Y, Zhou Y, Wang X, Qian W, Han X (2013) Microcystin-LR induces autophagy and apoptosis in rat Sertoli cells in vitro. Toxicon 76:84–93
Egan DF, Chun MGH, Vamos M, Zou HX, Rong J, Miller CJ, Lou HJ, Raveendra-Panickar D, Yang CC, Sheffler DJ, Teriete P, Asara JM, Turk BE, Cosford NDP, Shaw RJ (2015) Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol Cell 59:285–297
França LR, Hess RA, Dufour JM, Hofmann MC, Griswold MD (2016) The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology 4:189–212
Fulvio R, Klionsky DJ (2013) Autophagic processes in yeast: mechanism, machinery and regulation. Genetics 194:341–361
Ganley RJGJ, Watt MSWS, Manning LM, Iturritxa EI (2009) A global climatic risk assessment of pitch canker disease. Can J For Res 39:2246–2256
Griswold MD (1998) The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol 9:411–416
Hai Y, Hou J, Liu Y, Liu Y, Yang H, Li Z, He Z (2014) The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis. Semin Cell Dev Biol 29:66–75
Han L, Zhang G, Yang H, Pang J, Wan Y, Yao X, Wang F, Zhang Y (2018) Goat testis sertoli cell culture and autophagy dual-fluorescence reporter system construction. J Nanjing Agric Univ. https://doi.org/10.7685/jnau.201612007
Herbert Z, Weigel S, Sendemir E, Marshall A, Caldwell JD, Petrusz P, Peuckert C, Jirikowski GF (2005) Androgen-binding protein is co-expressed with oxytocin in the male reproductive tract. Anat Histol Embryol 34:286–293
Hess RA, Franca LRD (2008) Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 636:1
Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura SI, Natsume T, Takehana K, Yamada N (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20:1981–1991
Kyrönlahti A, Euler R, Bielinska M, Schoeller EL, Moley KH, Toppari J, Heikinheimo M, Wilson DB (2011) GATA4 regulates Sertoli cell function and fertility in adult male mice. Mol Cell Endocrinol 333:85–95
Lee EJ, Tournier C (2011) The requirement of uncoordinated 51-like kinase 1 (ULK1) and ULK2 in the regulation of autophagy. Autophagy 7:689–695
Li Y, Wang C, Zhang G, Wang X, Duan R, Gao H, Peng T, Teng J, Jia Y (2015) Role of autophagy and mTOR signaling in neural differentiation of bone marrow mesenchymal stem cells. Cell Biol Int 38:1337–1343
Ma Y, Yang HZ, Xu LM, Huang YR, Dai HL, Kang XN (2015) Testosterone regulates the autophagic clearance of androgen binding protein in rat Sertoli cells. Sci Rep 5:8894
Maiese K (2015) Stem cell guidance through the mechanistic target of rapamycin. World J Stem Cells 7:999
Mathew R, Kongara S, Beaudoin B, Karp CM, Bray K, Degenhardt K, Chen G, Jin S, White E (2007) Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev 21:1367–1381
Mizushima N (2010) The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 22:132–139
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326
Nazarko VY, Zhong Q (2013) ULK1 targets Beclin-1 in autophagy. Nat Cell Biol 15:727–728
Oliveira PF, Sousa M, Silva BM, Monteiro MP, Alves MG (2017) Obesity, energy balance and spermatogenesis. Reproduction 153:R173–R185
Pohl C, Jentsch S (2009) Midbody ring disposal by autophagy is a post-abscission event of cytokinesis. Nat Cell Biol 11:65–70
Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW (2007) A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med 204:25–31
Razaul KM, Kawanago H, Kadowaki M (2014) A quick signal of starvation induced autophagy: transcription versus post-translational modification of LC3. Anal Biochem 465:28–34
Rubinsztein DC, Mariño G, Kroemer G (2011) Autophagy and aging. Cell 146:682–695
SA M, DD L, TH W, JJ F (2001) Specific staining of Sertoli cell nuclei and evaluation of Sertoli cell number and proliferative activity in Meishan and White Composite boars during the neonatal period. Biol Reprod 64:689–695
Scholz M, Cinatl J (2005) Fas/FasL interaction: a novel immune therapy approach with immobilized biologicals. Med Res Rev 25:331–342
Suning C, Rehman SK, Wei Z, Aidong W, Libo Y, Jian Z (2010) Autophagy is a therapeutic target in anticancer drug resistance. Biochim Biophys Acta 1806:220–229
Tanwar PS, Kaneko-Tarui T, Zhang L, Rani P, Taketo MM, Teixeira J (2010) Constitutive WNT/beta-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol Reprod 82:422–432
Ureshino RP, Rocha KK, Lopes GS, Bincoletto C, Smaili SS (2014) Calcium signaling alterations, oxidative stress, and autophagy in aging. Antioxid Redox Signal 21:123–137
Wang S, Xia P, Rehm M, Fan Z (2015) Autophagy and cell reprogramming. Cell Mol Life Sci 72:1699–1713
Wei B, Huang Q, Huang S, Mai W, Zhong X (2016) Trichosanthin-induced autophagy in gastric cancer cell MKN-45 is dependent on reactive oxygen species (ROS) and NF-κB/p53 pathway. J Pharmacol Sci 131:77–83
Yefimova MG, Nadia M, Thomas H, Annie-Claire M, Jonathan C, Anaïs N, Jean-Luc W, Anne C, Michel P, Nicolas B (2013) A chimerical phagocytosis model reveals the recruitment by Sertoli cells of autophagy for the degradation of ingested illegitimate substrates. Autophagy 9:653–666
Zhang Y, Han L, Yang H, Pang J, Li P, Zhang G, Li F, Wang F (2017) Bisphenol A affects cell viability involved in autophagy and apoptosis in goat testis sertoli cell. Environ Toxicol Pharmacol 55:137–147
Zhiping X, Klionsky DJ (2007) Autophagosome formation: core machinery and adaptations. Nat Cell Biol 9:1102–1109
Acknowledgments
The authors sincerely thank all the members of their laboratory for technical assistance during the preparation of the study.
Funding
This study was financially supported by the Natural Science Foundation of Jiangsu Province (BK20161444) and Chinese Fundamental Research Funds for the Central Universities (KYDK201702).
Author information
Authors and Affiliations
Contributions
Jing Pang and Le Han were responsible for the research design, the performance of the whole experiment, analysis of the data, and drafting and revising the manuscript. Zifei Liu and Jian Zheng helped to perform the experiments and analyze the data. Jie Zhao made contribution to perform the experiments. And Yanli Zhang and Feng Wang contributed significantly to the manuscript preparation. All listed authors have made substantial contributions to the research and its publication.
Corresponding author
Ethics declarations
All experimental designs and procedures were conducted in accordance with the approved guidelines for Animal Experiments of Nanjing Agricultural University and approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University (Approval ID: SYXK2011-0036).
Additional information
Editor: Tetsuji Okamoto
Jing Pang and Le Han are co-first authors
Electronic supplementary material
Supplementary Figure 1
(DOCX 528 kb)
Supplementary Table 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Pang, J., Han, L., Liu, Z. et al. ULK1 affects cell viability of goat Sertoli cell by modulating both autophagy and apoptosis. In Vitro Cell.Dev.Biol.-Animal 55, 604–613 (2019). https://doi.org/10.1007/s11626-019-00371-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-019-00371-2