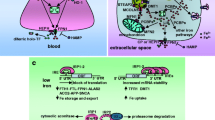
Abstract
Studies with patients, animal models of human disease and hemopexin null mice have shown that the heme-binding protein hemopexin is vital for the protection of a variety of cell types and tissues against heme toxicity. The presence of hemopexin in all biological fluids examined to date indicates wide roles in abrogating heme toxicity in human tissues; and, thus, is clinically relevant. Heme-hemopexin endocytosis leads to coordinated trafficking of heme, iron and copper as heme traffics from endosomes to heme oxygenases (HOs) in the smooth endoplasmic reticulum and to the nucleus. This is safe redox-metal trafficking, without oxidative stress, as iron released from heme catabolism by HOs as well as copper taken up with heme-hemopexin move through the cell. To our knowledge, this coordinated metal trafficking has been described only for the hemopexin system and differs from the cell’s response to non-protein bound heme, which can be toxic. We propose that defining how cells respond to heme-hemopexin endocytosis, a natural cytoprotective system, will aid our understanding of how cells adapt as they safely respond to increases in heme, Fe(II) and copper. This is relevant for many genetic hemolytic diseases and conditions, stroke and hemorrhage as well as neurodegeneration. Such analyses will help to define a pattern of events that can be utilized to characterize how dysfunctional redox and transition metal handling is linked to the development of pathology in disease states such as Alzheimer’s disease when metal homeostasis is not restored; and potentially provide novel targets and approaches to improve therapies.




Similar content being viewed by others
References
Acevedo KM et al (2011) Copper promotes the trafficking of the amyloid precursor protein. J Biol Chem 286:8252–8262. https://doi.org/10.1074/jbc.M110.128512
Alam J, Smith A (1989) Receptor-mediated transport of heme by hemopexin regulates gene expression in mammalian cells. J Biol Chem 264:17637–17640
Ayton S, Faux NG, Bush AI (2015) Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun 6:6760
Ayton S, Diouf I, Bush AI (2017a) Evidence that iron accelerates ALzheimer’s pathology: a CSF biomarker study. J Neurol Neurosurg Psychiatry 89:456–460
Ayton S et al (2017b) Cerebral quantitative susceptibility mapping predicts amyloid-beta-related congitive decline. Brain 140:2112–2119
Bai B et al (2013) U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer’s disease. Proc Natl Acad Sci USA 110:16562–16567. https://doi.org/10.1073/pnas.1310249110
Balla J, Jacob HS, Balla G, Nath K, Eaton JW, Vercellotti GM (1993) Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage Proc Natl Acad Sci USA 90:9285–9289
Belcher JD et al (2014) Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 123:377–390. https://doi.org/10.1182/blood-2013-04-495887
Bertinato J, L’Abbe MR (2003) Copper modulates the degradation of copper chaperone for Cu, Zn superoxide dismutase by the 26 S proteosome. J Biol Chem 278:35071–35078
Bertrand RL (2017) Iron accumulation, glutathione depletion, and lipid peroxidation must occur simultaneously during ferroptosis and are mutually amplifying events. Med Hypotheses 101:69–74
Castano EM, Roher AE, Esh CL, Kokjohn TA, Beach T (2006) Comparative proteomics of cerebrospinal fluid in neuropathologically-confirmed Alzheimer’s disease and non-demented elderly subjects. Neurol Res 28:155–163
Crapper McLachlan DR, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W, Andrews DF (1991) Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 337:1304–1308
Davies DM, Smith A, Muller-Eberhard U, Morgan WT (1979) Hepatic subcellular metabolism of heme from heme-hemopexin: incorporation of iron into ferritin. Biochem Biophys Res Commun 91:1504–1511
De Domenico I, Ward DM, Kaplan J (2011) Hepcidin and ferroportin: the new players in iron metabolism. Semin Liver Dis 31:272–279. https://doi.org/10.1055/s-0031-1286058
Delaby C, Pilard N, Puy H, Canonne-Hergaux F (2008) Sequential regulation of ferroportin expression after erythrophagocytosis in murine macrophages: early mRNA induction by haem, followed by iron-dependent protein expression. Biochem J 411:123–131
Demars MP, Bartholomew A, Strakova Z, Lazarov O (2011) Soluble amyloid precursor protein: a novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. Stem Cell Res Ther 2:36. https://doi.org/10.1186/scrt77
Drabkin DL (1971) Heme binding and transport-a spectrophotometric study of plasma glycoglobulin hemochromogens. Proc Natl Acad Sci USA 68:609–613
Duce JA et al (2010) Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell 142:857–867. https://doi.org/10.1016/j.cell.2010.08.014
Flaherty MM, Rish KR, Smith A, Crumbliss AL (2008) An investigation of hemopexin redox properties by spectroelectrochemistry: biological relevance for heme uptake. Biometals 21:239–248. https://doi.org/10.1007/s10534-007-9112-9
Grinshtein N, Bamm VV, Tsemakhovich VA, Shaklai N (2003) Mechanism of low-density lipoprotein oxidation by hemoglobin-derived iron. Biochemistry 42:6977–6985. https://doi.org/10.1021/bi020647r
Grzywacz A, Gdula-Argasinska J, Muszynska B, Tyszka-Czochara M, Librowski T, Opoka W (2015) Metal responsive transcription factor 1 (MTF-1) regulates zinc dependent cellular processes at the molecular level. Acta Biochim Pol 62:491–498. https://doi.org/10.18388/abp.2015_1038
Hahl P, Davis T, Washburn C, Rogers JT, Smith A (2013) Mechanisms of neuroprotection by hemopexin: modeling the control of heme and iron homeostasis in brain neurons in inflammatory states. J Neurochem 125:89–101. https://doi.org/10.1111/jnc.12165
Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK (2005) Identification of the receptor scavenging hemopexin-heme complexes. Blood 106:2572–2579
Ingoglia G et al (2017) Hemopexin counteracts systolic dysfunction induced by heme-driven oxidative stress. Free Radic Biol Med 108:452–464. https://doi.org/10.1016/j.freeradbiomed.2017.04.003
Janz DR, Bastarache JA, Sills G, Wickersham N, May AK, Bernard GR, Ware LB (2013) Association between haptoglobin, hemopexin and mortality in adults with sepsis. Crit Care 17:R272. https://doi.org/10.1186/cc13108
Jung JY, Kwak YH, Kim KS, Kwon WY, Suh GJ (2015) Change of hemopexin level is associated with the severity of sepsis in endotoxemic rat model and the outcome of septic patients. J Crit Care 30:525–530. https://doi.org/10.1016/j.jcrc.2014.12.009
Larsen R et al (2010) A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med 2:5171
Liang X, Lin T, Sun G, Beasley-Topliffe L, Cavaillon JM, Warren HS (2009) Hemopexin down-regulates LPS-induced proinflammatory cytokines from macrophages. J Leukoc Biol 86:229–235
Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR (1998) Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci 158:47–52
Mauk MR, Rosell FI, Lelj-Garolla B, Moore GR, Mauk AG (2005) Metal ion binding to human hemopexin. Biochemistry 44:1864–1871
Maurer I, Zierz S, Moller HJ (2000) A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging 21:455–462
McCarthy RC, Park YH, Kosman DJ (2014) sAPP modulates iron efflux from brain microvascular endothelial cells by stabilizing the ferrous iron exporter ferroportin. EMBO Rep 15:809–815. https://doi.org/10.15252/embr.201338064
Mehta NU, Grijalva V, Hama S, Wagner A, Navab M, Fogelman AM, Reddy ST (2016) Apolipoprotein E-/- mice lacking hemopexin develop increased atherosclerosis via mechanisms that include oxidative stress and altered macrophage function. Arterioscler Thromb Vasc Biol 36:1152–1163. https://doi.org/10.1161/ATVBAHA.115.306991
Miller YI, Shaklai N (1999) Kinetics of hemin distribution in plasma reveals its role in lipoprotein oxidation. Biochim Biophys Acta 1454:153–164
Nelson PT, Wang WX (2010) MiR-107 is reduced in Alzheimer’s disease brain neocortex: validation study. J Alzheimers Dis 21:75–79. https://doi.org/10.3233/JAD-2010-091603
Onyango IG, Dennis J, Khan SM (2016) Mitochondrial dysfunction in Alzheimer’s disease and the rationale for bioenergetics based therapies. Aging Dis 7:201–214
Parker WD Jr, Parks JK (1995) Cytochrome c oxidase in Alzheimer’s disease brain: purification and characterization. Neurology 45:482–486
Philpott CC, Ryu MS, Frey A, Patel S (2017) Cytosolic iron chaperones: proteins delivering iron cofactors in the cytosol of mammalian cells. J Biol Chem 292:12764–12771. https://doi.org/10.1074/jbc.R117.791962
Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Breteler MM (2010) Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke 41:S103–S106. https://doi.org/10.1161/STROKEAHA.110.595181
Ren Y, Smith A (1995) Mechanism of metallothionein gene regulation by heme-hemopexin. Roles of protein kinase C, reactive oxygen species, and cis-acting elements J Biol Chem 270:23988–23995
Roeb E et al (2002) The matrix metalloproteinase 9 (mmp-9) hemopexin domain is a novel gelatin binding domain and acts as an antagonist. J Biol Chem 277:50326–50332
Rogers JT et al (2008) Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer’s disease. Biochem Soc Trans 36:1282–1287
Rogers JT et al (2016) A role for amyloid precursor protein translation to restore iron homeostasis and ameliorate lead (Pb) neurotoxicity. J Neurochem 138:479–494. https://doi.org/10.1111/jnc.13671
Rosell FI, Mauk MR, Mauk AG (2005) pH- and metal ion-linked stability of the hemopexin-heme complex. Biochemistry 44:1872–1879
Smith A, Hunt RC (1990) Hemopexin joins transferrin as representative members of a distinct class of receptor-mediated endocytic transport systems. Eur J Cell Biol 53:234–245
Smith A, McCulloh RJ (2015) Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders Front Physiol 6:187. https://doi.org/10.3389/fphys.2015.00187
Smith A, Morgan WT (1979) Haem transport to the liver by haemopexin. Receptor-mediated uptake with recycling of the protein Biochem J 182:47–54
Smith A, Alam J, Escriba PV, Morgan WT (1993) Regulation of heme oxygenase and metallothionein gene expression by the heme analogs, cobalt-, and tin-protoporphyrin. J Biol Chem 268:7365–7371
Smith A, Rish KR, Lovelace R, Hackney JF, Helston RM (2009) Role for copper in the cellular and regulatory effects of heme-hemopexin. Biometals 22:421–437. https://doi.org/10.1007/s10534-008-9178-z
Sung L, Morales P, Shibata M, Shipulina N, Smith A (2000a) Defenses against extracellular heme-mediated oxidative damage: use of iron and copper chelators to investigate the role of redox active iron, copper and heme in the hemopexin heme transport system. In: Badman DG, Bergeron RJ, Brittenham GM (eds) Iron chelators: new development strategies. Saratoga Publishing Group, Sarotoga, pp 67–86
Sung L, Shibata M, Eskew JD, Shipulina N, Morales PJ, Smith A (2000b) Cell surface events for metallothionein-1 and heme oxygenase-1 regulation by the hemopexin-heme transport system. Antioxid Redox Signal 2:753–765
Swerdlow RH (2018) Mitochondria and mitochondrial cascades in alzheimer’s disease. J Alzheimers Dis 62:1403–1416. https://doi.org/10.3233/JAD-170585
Tapia L, Gonzalez-Aguero M, Cisternas MF, Suazo M, Cambiazo V, Uauy R, Gonzalez M (2004) Metallothionein is crucial for safe intracellular copper storage and cell survival at normal and supra-physiological exposure levels. Biochem J 378:617–624
Tonelli C, Chio IIC, Tuveson DA (2018) Transcriptional Regulation by Nrf2. Antioxid Redox Signal 29:1727–1745. https://doi.org/10.1089/ars.2017.7342
Tsemakhovich VA, Bamm VV, Shaklai M, Shaklai N (2005) Vascular damage by unstable hemoglobins: the role of heme-depleted globin. Arch Biochem Biophys 436:307–315
van Golen RF et al (2019) The damage-associated molecular pattern HMGB1 is released early after clinical hepatic ischemia/reperfusion. Biochim Biophys Acta Mol Basis Dis. https://doi.org/10.1016/j.bbadis.2019.01.014
Vanacore R, Eskew JD, Morales PJ, Sung L, Smith A (2000) Role for copper in transient oxidation and nuclear translocation of MTF-1, but not of NFkB, by the hemopexin heme transport system. Antioxid Redox Signal 2:739–752
Vercellotti GM et al (2016) Hepatic overexpression of hemopexin inhibits inflammation and vascular stasis in murine models of sickle cell disease. Mol Med 22:437–451. https://doi.org/10.2119/molmed.2016.00063
Vinchi F et al (2016) Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood 127:473–486. https://doi.org/10.1182/blood-2015-08-663245
Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A (2001) Sundowning and circadian rhythms in Alzheimer’s disease. Am J Psychiatry 158:704–711. https://doi.org/10.1176/appi.ajp.158.5.704
Wang WX et al (2008) The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci 28:1213–1223. https://doi.org/10.1523/JNEUROSCI.5065-07.2008
Wang B, Dong D, Kang YJ (2013) Copper chaperone for superoxide dismutase-1 transfers copper to mitochondria but does not affect cytochrome c oxidase activity. Exp Biol Med 238:1017–1023. https://doi.org/10.1177/1535370213497327
West EC, Prohaska JR (2004) Cu, Zn-superoxide dismutase is lower and copper chaperone CCS is higher in erythrocytes of copper-deficient rats and mice. Exp Biol Med 229:756–764
Acknowledgements
We acknowledge Drs. Peter Koulen and Jeffrey Price (School of Biological Sciences, University of Missouri-Kansas City) for help with the preparation of the manuscript, and Dr. Tamas Kapros (School of Biological Sciences, University of Missouri-Kansas City) for his help generating the final figures. We also acknowledge the expert technical help of members of the Smith research team Dr. Rachel M. Helston, Kimberley Rish, Rachel Hunt (formerly Lovelace) and Peter Hahl. This research was supported in part by the National Institutes of Health (R21 DK64363 to A.S.), by a grant from University of Missouri Research Board (A.S.) and Research Incentive Funds UMKC (A.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vanacore, R., Eskew, J.D., Sung, L. et al. Safe coordinated trafficking of heme and iron with copper maintain cell homeostasis: modules from the hemopexin system. Biometals 32, 355–367 (2019). https://doi.org/10.1007/s10534-019-00194-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-019-00194-4