Abstract
Background
To evaluate patient radiation dose and procedural duration recorded during pulmonary arteriovenous malformation (PAVM) embolisation performed using high-frequency jet ventilation (HFJV) as compared with conventional intermittent positive pressure ventilation (IPPV)
Methods
Patients undergoing PAVM embolisation with HFJV assistance after April 2017 were retrospectively identified as group A, and those treated with IPPV before April 2017 as group B. Primary outcomes were patient radiation dose and procedural duration between groups A and B. Secondary outcomes were difference in diaphragmatic excursion between groups A and B, in group A with/without HFJ assistance, technical/clinical success, and complications.
Results
Twelve PAVMs were embolised in 5 patients from group A, and 15 PAVMs in 10 patients from group B. Mean patient radiation was significantly lower in group A than in group B (54,307 ± 33,823 mGy cm2 [mean ± standard deviation] versus 100,704 ± 43,930 mGy cm2; p = 0.022). Procedural duration was 33.4 ± 16.1 min in group A versus 57.4 ± 14.9 min in group B (p = 0.062). Diaphragmatic excursion was significantly lower in group A (1.3 ± 0.4 mm) than in group B (19.7 ± 5.2 mm; p < 0.001) and lower with near statistical significance in group A with HFJV than without HFJV (1.3 ± 0.4 mm versus 10.9 ± 3.1 mm; p = 0.062). Technical and clinical success was 100% in both groups, without relevant complications.
Conclusion
HFJV-assisted PAVM embolisation is a safe, feasible technique resulting in reduced patient radiation doses and procedural time.
Similar content being viewed by others
Key points
-
Embolisation is the treatment of choice for pulmonary arteriovenous malformations.
-
Embolisation of pulmonary arteriovenous malformations can be performed under general anaesthesia and high-frequency jet ventilation.
-
This option results in reduced patient radiation dose and procedural time.
Background
Pulmonary arteriovenous malformations (PAVMs) are anomalous direct communications between pulmonary arteries and veins, comprising single/multiple feeding arteries (classified as “simple” or “complex” types, respectively), an aneurysmal sac/nidus, and one or more draining veins. Eighty per cent of them are associated with hereditary haemorrhagic telangiectasia (HHT), a rare autosomal-dominant disease characterised by recurrent epistaxis, mucocutaneous telangiectasia, and visceral arteriovenous malformations [1, 2]. PAVMs form a right-to-left shunt, potentially resulting in cyanosis, haemorrhage, and significant risk of paradoxical embolism (e.g. stroke, cerebral abscess) [3, 4]. Early treatment is usually recommended.
Endovascular embolisation is the treatment of choice and aims to selectively occlude feeding arteries as close as possible to the nidus, while avoiding device migration. Embolisation is typically performed under local anaesthesia and mild sedation. However, despite development of numerous devices facilitating safe, distal vascular occlusion (including microcatheters, detachable coils, microcoils, and vascular plugs [5,6,7,8,9,10,11,12,13]), procedures may remain challenging due to significant target-lesion motion during respiration. This may preclude two-dimensional (2D)/three-dimensional (3D) roadmap generation, limit precise device navigation/deployment to short periods of controlled apnoea or breathing intervals, and necessitate multiple angiographic acquisitions with increased procedure time and radiation dose. For these reasons, some operators may prefer general anaesthesia with intermittent positive pressure ventilation (IPPV).
High-frequency jet ventilation (HFJV) utilises limited tidal volumes (1–3 mL/kg, often less than dead space) at high frequencies (> 100 cycles/min) to minimise thoracoabdominal motion compared with IPPV [14, 15]. The technique has been applied to thoracic surgery, extracorporeal shockwave lithotripsy, cardiac ablation, and percutaneous tumour ablation procedures to reduce target-lesion motion and optimise therapy [14, 16,17,18,19,20,21,22,23,24,25]. To our knowledge, there are no prior studies evaluating potential benefits in endovascular embolisation procedures.
The purpose of this study is to evaluate patient radiation dose and procedural duration recorded during PAVM embolisation performed using HFJV as compared with conventional IPPV.
Methods
This retrospective study was approved by the institutional review board with a waiver of written informed consent.
Study population
A retrospective review of a prospectively maintained institutional database was performed to identify consecutive patients undergoing PAVM embolisation under general anaesthesia (mainly due to patients’ refusal of local anaesthesia) between December 2015 and May 2018. Following the introduction of HFJV at our institution in April 2017, all cases undergoing PAVM embolisation under general anaesthesia were systematically treated using HFJV assistance (group A); prior to this, patients undergoing PAVM embolisation under general anaesthesia were treated using conventional IPPV without HFJV (group B).
All patients were affected by HHT and referred for treatment following multidisciplinary discussion between pulmonologists, interventional radiologists, and anaesthesiologists, to prevent future cerebrovascular accident or ameliorate respiratory symptoms. All PAVMs were confirmed on echocardiography/bubble test, demonstrating right-to-left shunts, and contrast-enhanced computed tomography (CECT). No patients in group A had contraindications to HFJV (chronic airways disease with forced expiratory volume in the first second < 1.5, severe obesity, or recent pneumothorax/thoracic surgery).
Procedures
All procedures were performed on an in-patient basis by the same interventional radiologist (> 7 years of experience in embolisation) under sterile surgical conditions. Procedures were performed in an angiography suite equipped with a flat-panel C-arm cone-beam computed tomography (CBCT) system and XperCT/Embo-Guide tools (Philips Healthcare, Best, the Netherlands). Anticoagulant/anti-platelet therapy and blood clotting parameters were managed according to Society of Interventional Radiology guidelines [26]. Antibiotic prophylaxis (cefazolin, 1 g) and 5000 IU heparin were administered intravenously prior to and during the procedure, respectively.
Anaesthesia and HFJV assistance
Following induction of anaesthesia, muscle relaxation, and orotracheal intubation, conventional IPPV was commenced in all patients (6–8 mL/kg predicted body weight tidal volume; respiratory rate adjusted according to end-tidal carbon dioxide [ETCO2] level; target 30–40 mmHg). For group A patients, IPPV was discontinued and a HFJV dual-lumen cannula (length 40 cm, diameter 2 mm; Acutronic Medical Systems AG, Hirzel, Switzerland) was introduced into the endotracheal tube. HFJV was initiated using a Monsoon II jet ventilator (Acutronic Medical Systems AG, Hirzel, Switzerland) with frequency 150–250 breaths/min, driving pressure 1–2 bar, inspiratory time 30%, and fraction of inspired O2 (FiO2) adjusted to maintain peripheral oxygen saturation (SpO2) > 95%. Blood pressure, ECG, and SpO2 were continuously monitored. ETCO2 monitoring was performed intermittently after 5 min and then every 30 min using the jet ventilator capnography device, by injecting five long insufflations with IPPV followed by measurement over 10 s (with the jet switched off). Once embolisation was completed, the HFJV cannula was removed and conventional ventilation was resumed until patient’s awakening/extubation.
PAVM embolisation
Following ultrasound-guided placement of a 6–8-Fr sheath (Pinnacle, Terumo Corporation, Tokyo, Japan) in the femoral vein, a 4-Fr Imager TM II pig-tail catheter (Boston Scientific, Marlborough, MA, USA) was advanced into the target main pulmonary artery, and diagnostic angiography was performed to localise PAVMs and identify number/size of feeding arteries. Feeding arteries were selectively catheterised using a 6-Fr catheter (Neuron catheter, Penumbra Inc., Alameda, CA, USA), and when required, a co-axially 4-Fr MPA 125-cm catheter (Cordis, Johnson & Johnson Company, USA) was used. The 6-Fr catheter was continuously flushed with heparin-saline solution (2500 IU/L) via a Y connector to avoid thrombus formation and minimise neuro-embolic risk. For group A, navigation was assisted by a simple 2D roadmap (Fig. 1) in four patients, and a continuous 3D roadmap automatically delineating feeding arteries (generated by Embo-Guide software following initial 3D rotational angiography with 40 mL of contrast agent at 8 mL/s) was used in one patient. No 2D/3D roadmaps could be generated for group B. Embolisation was performed using conventional or microvascular plugs (MVP, Medtronic, Minneapolis, MN, USA) and/or platinum detachable microcoils with/without hydrogel coating (Ruby Coil, Penumbra Inc., Alameda, CA, USA; Azur Coils, Terumo Corporation, Tokyo, Japan), depending on feeding artery anatomy and available technologies during the study period.
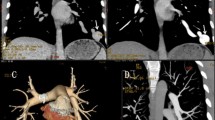
Fluoroscopic images from a group A patient undergoing HFJV-assisted embolisation. a Simple PAVM (white arrow) of the right lower lobe identified on initial angiogram. b 2D roadmap-assisted selective catheterisation of the feeding artery was performed. c Final angiographic check following embolisation with an 8-mm Amplatzer vascular plug IV demonstrates complete vascular occlusion. HFJV High-frequency jet ventilation, PAVM Pulmonary arteriovenous malformation
Devices were deployed as distally as possible within feeding arteries, via 4–6-Fr catheters for conventional vascular plugs, or via a co-axial 2.8-Fr microcatheter (Progreat, Terumo Corporation, Tokyo, Japan; inner diameter 0.027″) for microvascular plugs/microcoils. Vascular occlusion was confirmed via selective feeding artery angiogram. A final angiographic check was performed after 3–4 min to confirm satisfactory device position and occlusion. For group B, angiograms throughout the procedure were variably performed with induction of patient’s apnoea, depending on operator requirements. For group A, all angiograms were performed with HFJV, other than a final acquisition under conventional IPPV to compare differences in diaphragmatic excursion.
Following the procedure, patients were admitted to a recovery ward for 24-h observation and discharged when medically fit.
Follow-up
All patients were clinically reviewed by the treating interventional radiologist and underwent CECT to confirm PAVM occlusion 1 month post-procedure. Serial echocardiography/bubble tests were performed at 1, 6, and 12 months to monitor for right-to-left shunts.
Data collection and statistical analysis
Patients’ demographics; number, location, and type (simple or complex) of PAVMs treated per session; and type of embolic device were tabulated. Primary outcomes were patient radiation dose and procedural time. Secondary outcomes included mean intraprocedural diaphragmatic excursion, considered as a surrogate for whole-lung movement in group A versus B; mean diaphragmatic excursion in group A on final angiogram with HFJV versus IPPV; technical success; complications (according to the classification System of the Cardiovascular and Interventional Radiological Society of Europe [27]); and clinical success.
The patient radiation dose was calculated as dose-area product by the proprietary angiographic software (Philips Healthcare, Best, the Netherlands). Procedural time was calculated between the first and last angiographic acquisition.
Diaphragmatic excursion was calculated for each case on digital subtracted angiographic series (duration at least 15 s), measuring the cranio-caudal distance between the upper- and lower-most positions of the superimposed hemidiaphragmatic cupolas and recording the lowest measurement for each procedure (Fig. 2).
Measurements of diaphragmatic excursion within and between groups. a Diaphragmatic excursion (1.52 mm) measured in the cranio-caudal plane during the final angiographic check in a patient from group A under HFJV. b A larger measurement (8.57 mm) was obtained in the same patient during the same interventional session with IPPV. c Diaphragmatic excursion (11.76 mm) measured in a patient from group B under conventional IPPV. HFJV High-frequency jet ventilation, IPPV Intermittent positive pressure ventilation
Technical success was defined as distal occlusion of the feeding artery < 1 cm from the PAVM aneurysmal sac, with complete devascularisation on final angiogram. Clinical success was defined as the absence of neurologic/respiratory symptoms and echocardiographic right-to-left shunt at the last available clinical/sonographic follow-up.
Statistical analysis was performed using R software (R v3.4.5, R Foundation for Statistical Computing, Vienna, Austria). Non-parametric Wilcoxon test was used to compare numerical and discrete variables; p values < 0.05 were considered statistically significant.
Results
In the study period, 20 patients underwent PAVM embolisation. Among them, 15 were treated under general anaesthesia, thus representing the study population. In particular, between April 2017 and May 2018, 5 consecutive patients underwent PAVM embolisation using HFJV assistance (group A). Prior to this, 10 patients were treated using conventional IPPV without HFJV (group B). Mean number of PAVMs embolised per session was 2.4 ± 2.1 (range 1–6) in group A versus 1.5 ± 0.7 (range 1–3) in group B (p = 0.422). Patient and PAVM characteristics are summarised in Table 1.
Mean patient radiation exposure was significantly lower in group A than in group B: dose-area product 54,307 ± 33,823 mGy cm2 (mean ± standard deviation; range 6135–100,678 mGy cm2) versus 100,704 ± 43,930 mGy cm2 (range 53,109–176,634 mGy cm2), respectively (p = 0.022).
Procedural time was lower in group A than in group B with a trend towards statistical significance, 33.4 ± 16.1 min (range 16–55 min) versus 57.4 ± 14.9 min (range 41–85 min) (p = 0.062).
Mean diaphragmatic excursion was significantly lower in group A than in group B (1.3 ± 0.4 mm (range 0.9–1.9 mm) versus 19.7 ± 5.2 mm (range 8.7–27.1 mm); p < 0.001) and demonstrated a lower trend on final angiograms in group A with HFJV assistance than with IPPV (1.3 ± 0.4 mm (range 0.9–1.9 mm) versus 10.9 ± 3.1 mm (range 7.2–15 mm); p = 0.062).
Technical success was 100% in both groups without radiologic complications. One patient from group A experienced bronchospasm necessitating temporary switching from HFJV to conventional IPPV, but no episodes of desaturation, hypercapnia, or barotrauma (grade 1 complication) were recorded. All PAVMs were completely occluded on 1-month CECT follow-up without device migration. Clinical success was 100% in both groups on early follow-up (group A: mean 3.75 months, range 1–12 months; group B: mean 16.5 months, range 14–29 months).
Discussion
The HFJV is a mechanical ventilation method initially described in 1977 by Klain and Smith [28], in which small tidal volumes of pressurised gas are delivered through a narrow-bore endotracheal catheter at very high respiratory rates. The technique facilitates adequate gas exchange while minimising diaphragmatic excursion, and thoracoabdominal target lesions normally subject to significant respiratory positional variation are rendered relatively motionless [14, 15, 25]. Several recent interventional radiology reports have demonstrated improved lesion targeting, reduced technical difficulty and procedural time, and reduced patient radiation dose during thermal ablation of lung, liver, and renal tumours [19,20,21,22,23,24]. However, to our knowledge, the technique has not yet been reported for endovascular embolisation procedures.
In the present study, HFJV was applied to five cases undergoing PAVM embolisation. Patient radiation dose and procedural time were improved using HFJV compared with IPPV. The presence of nearly static PAVMs enabled generation of accurate 2D/3D roadmaps, facilitating easier catheter navigation and more precise, confident deployment of embolic material. Fewer angiographic acquisitions were required for lesion targeting, significantly reducing radiation dose by 54% and procedural time by mean 24 min (minus 42%, non-significant likely due to small sample size). Similar advantages have been described in prior thermal ablation studies [22,23,24].
These benefits are likely to be the result of the nearly static PAVMs, and for this reason, diaphragmatic excursion was compared with a historic series of 10 patients undergoing the same procedure using IPPV. As expected, HFJV-assisted cases demonstrated significantly lower diaphragmatic excursion compared to IPPV (mean 1.3 mm, compared with 20 mm for IPPV), and similar results (1.3 mm excursion with HFJV assistance compared with 11 mm for IPPV) were observed intraprocedurally in the five cases with HFJV assistance, although did not reach statistical significance likely due to small sample size. These measurements are comparable with reported excursion of 1–3 cm during quiet breathing and conventional IPPV [29, 30] and with prior studies reporting reduced diaphragmatic motion during HFJV-assisted extracorporeal shockwave lithotripsy [25].
Although direct, reliable/reproducible measurement of PAVM motion was not possible due to retrospective differences in projections/positioning between cases, diaphragmatic excursion was considered an adequate surrogate, since it was less prominently affected by interprocedural factors, and appears to be similar in magnitude to whole-lung movement during respiration [31]. The present measurement of 1.3 mm is comparable to prior reports of HFJV-assisted thermal ablation and radiotherapy procedures. Denys et al. [21] measured target tumour displacement during lung, liver, and renal tumour ablation using CT guidance and demonstrated a mean motion of 0.3 mm transversely and < 3.75 mm cranio-caudally. Biro et al. [20] reported a reduction in liver motion from 20 to 5 mm following switching from IPPV to HFJV, and liver motion < 3 mm has been reported during HFJV-assisted hepatic radiotherapy using fiducial markers [22]. Diaphragmatic excursion is therefore minimal compared with gross motion using standard IPPV tidal volumes [18], and HFJV assistance appears to provide effective respiratory immobilisation.
HFJV assistance appeared to be safe, with no episodes of desaturation, hypercapnia, or barotrauma. One patient experienced bronchospasm requiring temporary conversion to IPPV; however, this was due to insufficient depth of anaesthesia, and HFJV was promptly recommenced following additional anaesthetic administration. There were no differences in technical success/outcomes compared with IPPV. Use of current-generation systems (which continuously monitor airway pressure with an alarm signal to avoid barotrauma) and intermittent CO2 monitoring with HFJV further increases procedural safety [14]. HFJV also proved practical and widely applicable, with simple/user-friendly ventilator installation and few contra-indications (COPD with forced expiratory volume in the first second < 1500 mL, severe obesity, and recent pneumothorax/thoracic surgery [32]). However, the need for specially trained anaesthesiology teams, and potentially longer anaesthetic times are aspects that should be taken into account [14, 21, 23, 24].
Study limitations include small sample size, including only few patients undergoing PAVM embolisation under general anaesthesia, and short follow-up, limiting generalisability and outcome comparison. Groups were non-randomised, but there were no significant differences in demographics, lesions, and embolisation devices. Procedural duration did not include anaesthetic time, and there was no evaluation of anaesthesiologist technical difficulty due to the retrospective protocol. Finally, diaphragmatic excursion was used as a surrogate for PAVM motion and measured using a gross method. Although non-comparable to standardised diaphragmatic measurements and without proven correlation with PAVM motion, this was sufficient to illustrate significant relative motion reduction with HFJV assistance compared with IPPV.
In conclusion, in patients undergoing PAVM embolisation under general anaesthesia, HFJV-assisted embolisation is a safe, practical technique enabling respiratory immobilisation and improved lesion targeting thus resulting in reduced patient radiation dose and procedural time. Larger prospective randomised studies are required to confirm the short- and long-term effects of this resurgent anaesthetic technique in vascular interventional procedures.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- CBCT:
-
C-arm cone-beam CT
- CECT:
-
Contrast-enhanced computed tomography
- CT:
-
Computed tomography
- ETCO2 :
-
End-tidal carbon dioxide
- FiO2 :
-
Fraction of inspired oxygen
- HFJV:
-
High-frequency jet ventilation
- HHT:
-
Hereditary haemorrhagic telangiectasia
- IPPV:
-
Intermittent positive pressure ventilation
- MVP:
-
Microvascular plug
- PAVM:
-
Pulmonary arteriovenous malformation
- SpO2 :
-
Peripheral oxygen saturation
References
Lacombe P, Lacout A, Marcy PY et al (2013) Diagnosis and treatment of pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: an overview. Diagn Interv Imaging 94:835–848. https://doi.org/10.1016/j.diii.2013.03.014
Kjeldsen AD, Vase P, Green A (1999) Hereditary haemorrhagic telangiectasia: a population-based study of prevalence and mortality in Danish patients. J Intern Med 245:31–39. https://doi.org/10.1046/j.1365-2796.1999.00398.x
Babaker M, Breault S, Beigelman C, Lazor R, Aebischer N, Qanadli SD (2015) Endovascular treatment of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Swiss Med Wkly 145:w14151. https://doi.org/10.4414/smw.2015.14151
Cartin-Ceba R, Swanson KL, Krowka MJ (2013) Pulmonary arteriovenous malformations. Chest 144:1033–1044. https://doi.org/10.1378/chest.12-0924
Remy-Jardin M, Wattinne L, Remy J (1991) Transcatheter occlusion of pulmonary arterial circulation and collateral supply: failures, incidents, and complications. Radiology 180:699–705. https://doi.org/10.1148/radiology.180.3.1871280
Remy-Jardin M, Dumont P, Brillet PY, Dupuis P, Duhamel A, Remy J (2006) Pulmonary arteriovenous malformations treated with embolotherapy: helical CT evaluation of long-term effectiveness after 2–21-year follow-up. Radiology 239:576–585. https://doi.org/10.1148/radiol.2391050333
Pollak JS, Saluja S, Thabet A, Henderson KJ, Denbow N, White RI Jr (2006) Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radiol 17:35–44; quiz 45. https://doi.org/10.1097/01.RVI.0000191410.13974.B6
Letourneau-Guillon L, Faughnan ME, Soulez G et al (2010) Embolization of pulmonary arteriovenous malformations with amplatzer vascular plugs: safety and midterm effectiveness. J Vasc Interv Radiol 21:649–656. https://doi.org/10.1016/j.jvir.2010.01.026
Funaki B (2007) Embolization of pulmonary arteriovenous malformations. Semin Intervent Radiol 24:350–355. https://doi.org/10.1055/s-2007-985750
Dinkel HP, Triller J (2002) Pulmonary arteriovenous malformations: embolotherapy with superselective coaxial catheter placement and filling of venous sac with Guglielmi detachable coils. Radiology 223:709–714. https://doi.org/10.1148/radiol.2233010953
Abdel Aal AK, Ibrahim RM, Moustafa AS, Hamed MF, Saddekni S (2016) Persistence of pulmonary arteriovenous malformations after successful embolotherapy with Amplatzer vascular plug: long-term results. Diagn Interv Radiol 22:358–364. https://doi.org/10.5152/dir.2015.15262
Boatta E, Jahn C, Canuet M et al (2017) Pulmonary arteriovenous malformations embolized using a microvascular plug system: technical note on a preliminary experience. Cardiovasc Intervent Radiol 40:296–301. https://doi.org/10.1007/s00270-016-1493-0
Trerotola SO, Pyeritz RE (2010) Does use of coils in addition to amplatzer vascular plugs prevent recanalization? AJR Am J Roentgenol 195:766–771. https://doi.org/10.2214/AJR.09.3953
Galmén K, Harbut P, Freedman J, Jakobsson JG (2017) The use of high-frequency ventilation during general anaesthesia: an update. F1000Res 6:756. https://doi.org/10.12688/f1000research.10823.1
Evans E, Biro P, Bedforth N (2007) Jet ventilation. Contin Educ Anaesth Crit Care Pain [BJA Education] 7:2–5. https://doi.org/10.1093/bjaceaccp/mkl061
Mucksavage P, Mayer WA, Mandel JE, Van Arsdalen KN (2010) High-frequency jet ventilation is beneficial during shock wave lithotripsy utilizing a newer unit with a narrower focal zone. Can Urol Assoc J 4:333–335. https://doi.org/10.5489/cuaj.09160
Fritz P, Kraus HJ, Muhlnickel W, Sassmann V, Hering W, Strauch K (2010) High-frequency jet ventilation for complete target immobilization and reduction of planning target volume in stereotactic high single-dose irradiation of stage I non-small cell lung cancer and lung metastases. Int J Radiat Oncol Biol Phys 78:136–142. https://doi.org/10.1016/j.ijrobp.2009.07.1678
Raiten J, Elkassabany N, Mandel JE (2012) The use of high-frequency jet ventilation for out of operating room anesthesia. Curr Opin Anaesthesiol 25:482–485. https://doi.org/10.1097/ACO.0b013e3283554375
Galmen K, Harbut P, Freedman J, Jakobsson JG (2017) High frequency jet ventilation for motion management during ablation procedures, a narrative review. Acta Anaesthesiol Scand 61:1066–1074. https://doi.org/10.1111/aas.12950
Biro P, Spahn DR, Pfammatter T (2009) High-frequency jet ventilation for minimizing breathing-related liver motion during percutaneous radiofrequency ablation of multiple hepatic tumours. Br J Anaesth 102:650–653. https://doi.org/10.1093/bja/aep051
Denys A, Lachenal Y, Duran R, Chollet-Rivier M, Bize P (2014) Use of high-frequency jet ventilation for percutaneous tumor ablation. Cardiovasc Intervent Radiol 37:140–146. https://doi.org/10.1007/s00270-013-0620-4
Abderhalden S, Biro P, Hechelhammer L, Pfiffner R, Pfammatter T (2011) CT-guided navigation of percutaneous hepatic and renal radiofrequency ablation under high-frequency jet ventilation: feasibility study. J Vasc Interv Radiol 22:1275–1278. https://doi.org/10.1016/j.jvir.2011.04.013
Chung DY, Tse DM, Boardman P et al (2014) High-frequency jet ventilation under general anesthesia facilitates CT-guided lung tumor thermal ablation compared with normal respiration under conscious analgesic sedation. J Vasc Interv Radiol 25:1463–1469. https://doi.org/10.1016/j.jvir.2014.02.026
Buchan T, Walkden M, Jenkins K, Sultan P, Bandula S (2018) High-frequency jet ventilation during cryoablation of small renal tumours. Cardiovasc Intervent Radiol 41:1067–1073. https://doi.org/10.1007/s00270-018-1921-4
Canty DJ, Dhara SS (2009) High frequency jet ventilation through a supraglottic airway device: a case series of patients undergoing extra-corporeal shock wave lithotripsy. Anaesthesia 64:1295–1298. https://doi.org/10.1111/j.1365-2044.2009.06079.x
Sacks D, McClenny TE, Cardella JF, Lewis CA (2003) Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202. https://doi.org/10.1097/01.RVI.0000094584.83406.3e
Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL (2017) CIRSE quality assurance document and standards for classification of complications: the CIRSE classification system. Cardiovasc Intervent Radiol 40:1141–1146. https://doi.org/10.1007/s00270-017-1703-4
Klain M, Smith RB (1977) High frequency percutaneous transtracheal jet ventilation. Crit Care Med 5:280–287. https://doi.org/10.1097/00003246-197711000-00007
Mageras GS, Yorke E, Rosenzweig K et al (2001) Fluoroscopic evaluation of diaphragmatic motion reduction with a respiratory gated radiotherapy system. J Appl Clin Med Phys 2:191–200. https://doi.org/10.1120/1.1409235
Kleinman BS, Frey K, VanDrunen M et al (2002) Motion of the diaphragm in patients with chronic obstructive pulmonary disease while spontaneously breathing versus during positive pressure breathing after anesthesia and neuromuscular blockade. Anesthesiology 97:298–305. https://doi.org/10.1097/00000542-199809230-00008
Korreman SS (2015) Image-guided radiotherapy and motion management in lung cancer. Br J Radiol 88:20150100. https://doi.org/10.1259/bjr.20150100
Bourgain JL, Chollet M, Fischler M, Gueret G, Mayne A, membres du conseil du club en anesthesie en ORL (2010) Guide for the use of jet-ventilation during ENT and oral surgery. Ann Fr Anesth Reanim 29:720–727. https://doi.org/10.1016/j.annfar.2010.06.020
Funding
This paper received no funding.
Author information
Authors and Affiliations
Contributions
EB and RLC conceived, wrote, and supervised this paper. PDM, JG, MC, and BH collected the data. PDM analysed the data. NR, TMB, ML, CJ, and AG revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional review board approval has been obtained for the present study (Hôpitaux Universitaires de Strasbourg; 2018).
Consent for publication
Consent for publication was obtained from the enrolled patients.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Boatta, E., Cazzato, R.L., De Marini, P. et al. Embolisation of pulmonary arteriovenous malformations using high-frequency jet ventilation: benefits of minimising respiratory motion. Eur Radiol Exp 3, 26 (2019). https://doi.org/10.1186/s41747-019-0103-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41747-019-0103-8