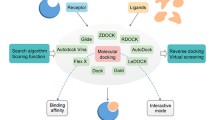
Abstract
Currently, molecular docking is becoming a key tool in drug discovery and molecular modeling applications. The reliability of molecular docking depends on the accuracy of the adopted scoring function, which can guide and determine the ligand poses when thousands of possible poses of ligand are generated. The scoring function can be used to determine the binding mode and site of a ligand, predict binding affinity and identify the potential drug leads for a given protein target. Despite intensive research over the years, accurate and rapid prediction of protein–ligand interactions is still a challenge in molecular docking. For this reason, this study reviews four basic types of scoring functions, physics-based, empirical, knowledge-based, and machine learning-based scoring functions, based on an up-to-date classification scheme. We not only discuss the foundations of the four types scoring functions, suitable application areas and shortcomings, but also discuss challenges and potential future study directions.




Similar content being viewed by others
Abbreviations
- SF:
-
Scoring function
- QM:
-
Quantum mechanics
- MM:
-
Molecular mechanics
- SVM:
-
Support vector machine
- RF:
-
Random forest
- ANN:
-
Artificial neural network
- DL:
-
Deep learning
- DNN:
-
Deep neural networks
References
Irwin JJ, Lorber DM, Mcgovern SL, Wei B, Shoichet BK (2002) Molecular docking and drug design. Comput Nanosci Nanotechnol 2:50–51
Fenu LALR, Good AC, Bodkin M, Essex JW (2007) Structure-based drug discovery. Springer, Dordrecht, p 24
Huang SY, Grinter SZ, Zou X (2010) Scoring functions and their evaluation methods for protein–ligand docking: recent advances and future directions. Phys Chem Chem Phys PCCP 12(40):12899–12908. https://doi.org/10.1039/c0cp00151a
Brooijmans N, Kuntz ID (2003) Molecular recognition and docking algorithms. Annu Rev Biophys Biomol Struct 32:335–373. https://doi.org/10.1146/annurev.biophys.32.110601.142532
Wang JC, Lin JH (2013) Scoring functions for prediction of protein–ligand interactions. Curr Pharm Design 19(12):2174–2182
Hermann JC, Marti-Arbona R, Fedorov AA, Fedorov E, Almo SC, Shoichet BK, Raushel FM (2007) Structure-based activity prediction for an enzyme of unknown function. Nature 448(7155):775–779
Joseph-Mccarthy D, Baber JC, Feyfant E, Thompson DC, Humblet C (2007) Lead optimization via high-throughput molecular docking. Curr Opin Drug Discov Devel 10(3):264–274
Jorgensen WL (2009) Efficient drug lead discovery and optimization. Acc Chem Res 42(6):724–733
Seifert MH, Kraus J, Kramer B (2007) Virtual high-throughput screening of molecular databases. Curr Opin Drug Discov Devel 10(3):298–307
Schneider G (2010) Virtual screening: an endless staircase? Nat Rev Drug Discov 9(4):273–276. https://doi.org/10.1038/nrd3139
Ain QU, Aleksandrova A, Roessler FD, Ballester PJ (2015) Machine-learning scoring functions to improve structure-based binding affinity prediction and virtual screening. Wiley Interdiscip Rev Comput Mol Sci 5(6):405–424. https://doi.org/10.1002/wcms.1225
Khamis MA, Gomaa W, Ahmed WF (2015) Machine learning in computational docking. Artif Intell Med 63(3):135–152. https://doi.org/10.1016/j.artmed.2015.02.002
Liu J, Wang R (2015) Classification of current scoring functions. J Chem Inf Model 55(3):475
Meng EC, Shoichet BK, Kuntz ID (1992) Automated docking with grid-based energy evaluation, vol 13. Wiley, New York
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79(2):926–935
Pullman B (1981) Intermolecular forces. D. Reidel Publishing Company, Dordrecht
Raha K, Peters MB, Wang B, Yu N, Wollacott AM, Westerhoff LM, Merz KM Jr (2007) The role of quantum mechanics in structure-based drug design. Drug Discov Today 12(17–18):725–731. https://doi.org/10.1016/j.drudis.2007.07.006
Senn HM, Thiel W (2009) QM/MM methods for biomolecular systems. Angew Chem (Int Edn Engl) 48(7):1198–1229. https://doi.org/10.1002/anie.200802019
Kramer B, Rarey M, Lengauer T (1999) Evaluation of the FLEXX incremental construction algorithm for protein–ligand docking. Proteins Struct Funct Bioinf 37(2):228–241
Yang Y, Lightstone FC, Wong SE (2013) Approaches to efficiently estimate solvation and explicit water energetics in ligand binding: the use of WaterMap. Expert Opin Drug Discov 8(3):277–287. https://doi.org/10.1517/17460441.2013.749853
Michel J, Tirado-Rives J, Jorgensen WL (2009) Prediction of the water content in protein binding sites. J Phys Chem B 113(40):13337–13346. https://doi.org/10.1021/jp9047456
Ross GA, Morris GM, Biggin PC (2012) Rapid and accurate prediction and scoring of water molecules in protein binding sites. PLoS One 7(3):e32036. https://doi.org/10.1371/journal.pone.0032036
Uehara S, Tanaka S (2016) AutoDock-GIST: incorporating thermodynamics of active-site water into scoring function for accurate protein–ligand docking. Molecules. https://doi.org/10.3390/molecules21111604
Kumar A, Zhang KY (2013) Investigation on the effect of key water molecules on docking performance in CSARdock exercise. J Chem Inf Model 53(8):1880–1892
Sun H, Li Y, Li D, Hou T (2013) Insight into crizotinib resistance mechanisms caused by three mutations in ALK tyrosine kinase using free energy calculation approaches. J Chem Inf Model 53(9):2376–2389. https://doi.org/10.1021/ci400188q
Sun HY, Hou TJ, Zhang HY (2014) Finding chemical drugs for genetic diseases. Drug Discov Today 19(12):1836–1840. https://doi.org/10.1016/j.drudis.2014.09.013
Chen F, Liu H, Sun H, Pan P, Li Y, Li D, Hou T (2016) Assessing the performance of the MM/PBSA and MM/GBSA methods. 6. Capability to predict protein–protein binding free energies and re-rank binding poses generated by protein–protein docking. Phys Chem Chem Phys PCCP 18(32):22129–22139. https://doi.org/10.1039/c6cp03670h
Kulik HJ (2018) Large-scale QM/MM free energy simulations of enzyme catalysis reveal the influence of charge transfer. Phys Chem Chem Phys PCCP 20(31):20650–20660. https://doi.org/10.1039/c8cp03871f
Orozco-Gonzalez Y, Manathunga M, Marin MDC, Agathangelou D, Jung KH, Melaccio F, Ferre N, Haacke S, Coutinho K, Canuto S, Olivucci M (2017) An average solvent electrostatic configuration protocol for QM/MM free energy optimization: implementation and application to rhodopsin systems. J Chem Theory Comput 13(12):6391–6404. https://doi.org/10.1021/acs.jctc.7b00860
Chaskar P, Zoete V, Röhrig UF (2017) On-the-Fly QM/MM docking with attracting cavities. J Chem Inf Model 57(1):73–84. https://doi.org/10.1021/acs.jcim.6b00406
Natesan S, Subramaniam R, Bergeron C, Balaz S (2012) Binding affinity prediction for ligands and receptors forming tautomers and ionization species: inhibition of mitogen-activated protein kinase-activated protein kinase 2 (MK2). J Med Chem 55(5):2035–2047. https://doi.org/10.1021/jm201217q
Chaskar P, Zoete V, Rohrig UF (2014) Toward on-the-fly quantum mechanical/molecular mechanical (QM/MM) docking: development and benchmark of a scoring function. J Chem Inf Model 54(11):3137–3152. https://doi.org/10.1021/ci5004152
Steinmann C, Olsson MA, Ryde U (2018) Relative ligand-binding free energies calculated from multiple short QM/MM MD simulations. Acs Nano 14(6):3228–3237
Eldridge MD, Murray CW, Auton TR, Paolini GV, Mee RP (1997) Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J Comput Aided Mol Design 11(5):425–445
Murray CW, Auton TR, Eldridge MD (1998) Empirical scoring functions. II. The testing of an empirical scoring function for the prediction of ligand-receptor binding affinities and the use of Bayesian regression to improve the quality of the model. J Comput Aided Mol Design 12(5):503–519
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT (2006) Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein–ligand complexes. J Med Chem 49(21):6177–6196. https://doi.org/10.1021/jm051256o
Zheng Z, Merz KM (2011) Ligand identification scoring algorithm (LISA). J Chem Inf Model 51(6):1296–1306. https://doi.org/10.1021/ci2000665
Kadukova M, Grudinin S (2017) Convex-PL: a novel knowledge-based potential for protein–ligand interactions deduced from structural databases using convex optimization. J Comput Aided Mol Design 31(10):943–958. https://doi.org/10.1007/s10822-017-0068-8
Fornabaio M, Spyrakis F, Mozzarelli A, Cozzini P, Abraham DJ, Kellogg GE (2004) Simple, intuitive calculations of free energy of binding for protein–ligand complexes. 3. The free energy contribution of structural water molecules in HIV-1 protease complexes. J Med Chem 47(18):4507–4516. https://doi.org/10.1021/jm030596b
Kerzmann A, Neumann D, Kohlbacher O (2006) SLICK—scoring and energy functions for protein–carbohydrate interactions. J Chem Inf Model 46(4):1635–1642. https://doi.org/10.1021/ci050422y
Catana CS, Novel PFW (2007) Customizable scoring functions, parameterized using N-PLS, for structure-based drug discovery. J Chem Inf Model 47(1):85–91
Sotriffer CA, Sanschagrin P, Matter H, Klebe G (2008) SFCscore: scoring functions for affinity prediction of protein–ligand complexes. Proteins 73(2):395–419. https://doi.org/10.1002/prot.22058
Bohm HJ (1994) The development of a simple empirical scoring function to estimate the binding constant for a protein–ligand complex of known three-dimensional structure. J Comput Aided Mol Design 8(3):243–256
Jain AN (2003) Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine. J Med Chem 46(4):499–511. https://doi.org/10.1021/jm020406h
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461. https://doi.org/10.1002/jcc.21334
Li Y, Liu Z, Li J, Han L, Liu J, Zhao Z, Wang R (2014) Comparative assessment of scoring functions on an updated benchmark: 1. Compilation of the test set. J Chem Inf Model 54(6):1700–1716. https://doi.org/10.1021/ci500080q
Thornton BF, Wik M, Crill PM (2017) Double-counting challenges the accuracy of high-latitude methane inventories. Geophys Res Lett 43:(24)
Muegge I, Martin YC (1999) A general and fast scoring function for protein–ligand interactions: a simplified potential approach. J Med Chem 42(5):791–804. https://doi.org/10.1021/jm980536j
Gohlke H, Hendlich M, Klebe G (2000) Knowledge-based scoring function to predict protein–ligand interactions. J Mol Biol 295(2):337–356. https://doi.org/10.1006/jmbi.1999.3371
Velec HF, Gohlke H, Klebe G (2005) DrugScore(CSD)-knowledge-based scoring function derived from small molecule crystal data with superior recognition rate of near-native ligand poses and better affinity prediction. J Med Chem 48(20):6296–6303. https://doi.org/10.1021/jm050436v
Neudert G, Klebe G (2011) DSX: a knowledge-based scoring function for the assessment of protein–ligand complexes. J Chem Inf Model 51(10):2731–2745. https://doi.org/10.1021/ci200274q
Mooij WT, Verdonk ML (2005) General and targeted statistical potentials for protein–ligand interactions. Proteins 61(2):272–287. https://doi.org/10.1002/prot.20588
Yang CY, Wang R, Wang S (2006) M-score: a knowledge-based potential scoring function accounting for protein atom mobility. J Med Chem 49(20):5903–5911. https://doi.org/10.1021/jm050043w
Huang SY, Zou X (2006) An iterative knowledge-based scoring function to predict protein–ligand interactions: I. Derivation of interaction potentials. J Comput Chem 27(15):1866–1875. https://doi.org/10.1002/jcc.20504
Huang SY, Zou X (2006) An iterative knowledge-based scoring function to predict protein–ligand interactions: II. Validation of the scoring function. J Comput Chem 27(15):1876–1882. https://doi.org/10.1002/jcc.20505
Huang SY, Zou X (2014) A knowledge-based scoring function for protein–RNA interactions derived from a statistical mechanics-based iterative method. Nucleic Acids Res 42(7):e55. https://doi.org/10.1093/nar/gku077
Forli S, Olson AJ (2012) A force field with discrete displaceable waters and desolvation entropy for hydrated ligand docking. J Med Chem 55(2):623–638. https://doi.org/10.1021/jm2005145
Huang SY, Zou X (2010) Inclusion of solvation and entropy in the knowledge-based scoring function for protein–ligand interactions. J Chem Inf Model 50(2):262–273. https://doi.org/10.1021/ci9002987
Lu M, Dousis AD, Ma J (2008) OPUS-PSP: an orientation-dependent statistical all-atom potential derived from side-chain packing. J Mol Biol 376(1):288–301. https://doi.org/10.1016/j.jmb.2007.11.033
Xu G, Ma T, Zang T, Sun W, Wang Q, Ma J (2017) OPUS-DOSP: a distance- and orientation-dependent all-atom potential derived from side-chain packing. J Mol Biol 429(20):3113–3120. https://doi.org/10.1016/j.jmb.2017.08.013
Li Y, Han L, Liu Z, Wang R (2014) Comparative assessment of scoring functions on an updated benchmark: 2. Evaluation methods and general results. J Chem Inf Model 54(6):1717–1736. https://doi.org/10.1021/ci500081m
Park J, Saitou K (2014) ROTAS: a rotamer-dependent, atomic statistical potential for assessment and prediction of protein structures. BMC Bioinform 15:307. https://doi.org/10.1186/1471-2105-15-307
Zheng Z, Merz KM Jr (2013) Development of the knowledge-based and empirical combined scoring algorithm (KECSA) to score protein–ligand interactions. J Chem Inf Model 53(5):1073–1083. https://doi.org/10.1021/ci300619x
Ma DL, Chan DS, Leung CH (2013) Drug repositioning by structure-based virtual screening. Chem Society Rev 42(5):2130–2141. https://doi.org/10.1039/c2cs35357a
Cheng T, Li Q, Zhou Z, Wang Y, Bryant SH (2012) Structure-based virtual screening for drug discovery: a problem-centric review. AAPS J 14(1):133–141. https://doi.org/10.1208/s12248-012-9322-0
Zhang L, Ai HX, Li SM, Qi MY, Zhao J, Zhao Q, Liu HS (2017) Virtual screening approach to identifying influenza virus neuraminidase inhibitors using molecular docking combined with machine-learning-based scoring function. Oncotarget 8(47):83142–83154. https://doi.org/10.18632/oncotarget.20915
Zhang L, Qiao M, Gao H, Hu B, Tan H, Zhou X, Li CM (2016) Investigation of mechanism of bone regeneration in a porous biodegradable calcium phosphate (CaP) scaffold by a combination of a multi-scale agent-based model and experimental optimization/validation. Nanoscale 8(31):14877–14887. https://doi.org/10.1039/c6nr01637e
Zhang L, Zhang S (2017) Using game theory to investigate the epigenetic control mechanisms of embryo development: Comment on: “Epigenetic game theory: How to compute the epigenetic control of maternal-to-zygotic transition” by Qian Wang et al. Phys Life Rev 20:140–142. https://doi.org/10.1016/j.plrev.2017.01.007
Zhang L, Zheng CQ, Li T, Xing L, Zeng H, Li TT, Yang H, Cao J, Chen BD, Zhou ZY (2017) Building up a robust risk mathematical platform to predict colorectal cancer. Complexity 2017:14. https://doi.org/10.1155/2017/8917258
Kinnings SL, Liu N, Tonge PJ, Jackson RM, Xie L, Bourne PE (2011) A machine learning-based method to improve docking scoring functions and its application to drug repurposing. J Chem Inf Model 51(2):408–419. https://doi.org/10.1021/ci100369f
Brylinski M (2013) Nonlinear scoring functions for similarity-based ligand docking and binding affinity prediction. J Chem Inf Model 53(11):3097–3112. https://doi.org/10.1021/ci400510e
Li GB, Yang LL, Wang WJ, Li LL, Yang SY (2013) ID-Score: a new empirical scoring function based on a comprehensive set of descriptors related to protein–ligand interactions. J Chem Inf Model 53(3):592–600. https://doi.org/10.1021/ci300493w
Koppisetty CA, Frank M, Kemp GJ, Nyholm PG (2013) Computation of binding energies including their enthalpy and entropy components for protein–ligand complexes using support vector machines. J Chem Inf Model 53(10):2559–2570. https://doi.org/10.1021/ci400321r
Ding B, Li N, Wang W (2013) Characterizing binding of small molecules. II. Evaluating the potency of small molecules to combat resistance based on docking structures. J Chem Inf Model 53(5):1213–1222. https://doi.org/10.1021/ci400011c
Ding B, Wang J, Li N, Wang W (2013) Characterization of small molecule binding. I. Accurate identification of strong inhibitors in virtual screening. J Chem Inf Model 53(1):114–122. https://doi.org/10.1021/ci300508m
Yan Y, Wang W, Sun Z, Zhang JZH, Ji C (2017) Protein–ligand empirical interaction components for virtual screening. J Chem Inf Model 57(8):1793–1806. https://doi.org/10.1021/acs.jcim.7b00017
Li H, Leung KS, Wong MH, Ballester PJ (2014) Substituting random forest for multiple linear regression improves binding affinity prediction of scoring functions: cyscore as a case study. BMC Bioinf 15:291. https://doi.org/10.1186/1471-2105-15-291
Afifi K, Al-Sadek AF (2018) Improving classical scoring functions using random forest: the non-additivity of free energy terms’ contributions in binding. Chem Biol Drug Design. https://doi.org/10.1111/cbdd.13206
Wang C, Zhang Y (2017) Improving scoring-docking-screening powers of protein–ligand scoring functions using random forest. J Comput Chem 38(3):169–177. https://doi.org/10.1002/jcc.24667
Zilian D, Sotriffer CA (2013) SFCscore(RF): a random forest-based scoring function for improved affinity prediction of protein–ligand complexes. J Chem Inf Model 53(8):1923–1933. https://doi.org/10.1021/ci400120b
Liu Q, Kwoh CK, Li J (2013) Binding affinity prediction for protein–ligand complexes based on beta contacts and B factor. J Chem Inf Model 53(11):3076–3085. https://doi.org/10.1021/ci400450h
Ballester PJ, Mitchell JBO (2010) A machine learning approach to predicting protein–ligand binding affinity with applications to molecular docking. Oxford University Press, Oxford
Ballester PJ, Schreyer A, Blundell TL (2014) Does a more precise chemical description of protein–ligand complexes lead to more accurate prediction of binding affinity? J Chem Inf Model 54(3):944–955. https://doi.org/10.1021/ci500091r
Li H, Leung KS, Wong MH, Ballester PJ (2015) Improving AutoDock Vina using random forest: the growing accuracy of binding affinity prediction by the effective exploitation of larger data sets. Mol Inf 34(2–3):115–126. https://doi.org/10.1002/minf.201400132
Gabel J, Desaphy J, Rognan D (2014) Beware of machine learning-based scoring functions—on the danger of developing black boxes. J Chem Inf Model 54(10):2807–2815. https://doi.org/10.1021/ci500406k
Cang Z, Mu L, Wei GW (2018) Representability of algebraic topology for biomolecules in machine learning based scoring and virtual screening. PLoS Comput Biol 14(1):e1005929. https://doi.org/10.1371/journal.pcbi.1005929
Buiu C, Putz MV, Avram S (2016) Learning the relationship between the primary structure of HIV envelope glycoproteins and neutralization activity of particular antibodies by using artificial neural networks. Int J Mol Sci. https://doi.org/10.3390/ijms17101710
Winkler DA, Burden FR (2007) Nonlinear predictive modeling of MHC class II-peptide binding using Bayesian neural networks. Methods Mol Biol (Clifton NJ) 409:365–377. https://doi.org/10.1007/978-1-60327-118-9_27
Fabry-Asztalos L, Andonie R, Collar CJ, Abdul-Wahid S, Salim N (2008) A genetic algorithm optimized fuzzy neural network analysis of the affinity of inhibitors for HIV-1 protease. Bioorg Med Chem 16(6):2903–2911. https://doi.org/10.1016/j.bmc.2007.12.055
Shen J, Cui Y, Gu J, Li Y, Li L (2014) A genetic algorithm-back propagation artificial neural network model to quantify the affinity of flavonoids toward P-glycoprotein. Combinatorial Chem High Throughput Screen 17(2):162–172
O’Donnell TJ, Rubinsteyn A, Bonsack M, Riemer AB, Laserson U, Hammerbacher J (2018) MHCflurry: open-source class I MHC binding affinity prediction. Cell Syst 7(1):129–132.e124. https://doi.org/10.1016/j.cels.2018.05.014
Chupakhin V, Marcou G, Baskin I, Varnek A, Rognan D (2013) Predicting ligand binding modes from neural networks trained on protein–ligand interaction fingerprints. J Chem Inf Model 53(4):763–772. https://doi.org/10.1021/ci300200r
Durrant JD, Mccammon JA (2010) NNScore: a neural-network-based scoring function for the characterization of protein–ligand complexes. J Chem Inf Model 50(10):1865–1871
Durrant JD, McCammon JA (2011) NNScore 2.0: a neural-network receptor-ligand scoring function. J Chem Inf Model 51(11):2897–2903. https://doi.org/10.1021/ci2003889
Durrant JD, Friedman AJ, Rogers KE, McCammon JA (2013) Comparing neural-network scoring functions and the state of the art: applications to common library screening. J Chem Inf Model 53(7):1726–1735. https://doi.org/10.1021/ci400042y
Ashtawy HM, Mahapatra NR (2018) Boosted neural networks scoring functions for accurate ligand docking and ranking. J Bioinf Comput Biol 16(2):1850004. https://doi.org/10.1142/s021972001850004x
Ashtawy HM, Mahapatra NR (2015) BgN-Score and BsN-Score: bagging and boosting based ensemble neural networks scoring functions for accurate binding affinity prediction of protein–ligand complexes. BMC Bioinf 16(Suppl 4):S8. https://doi.org/10.1186/1471-2105-16-s4-s8
Wallach I, Dzamba M, Heifets A (2015) AtomNet: a deep convolutional neural network for bioactivity prediction in structure-based drug discovery. Math Z 47(1):34–46
Ragoza M, Hochuli J, Idrobo E, Sunseri J, Koes DR (2017) Protein–ligand scoring with convolutional neural networks. J Chem Inf Model 57(4):942–957. https://doi.org/10.1021/acs.jcim.6b00740
Gomes J, Ramsundar B, Feinberg EN, Pande VS (2017) Atomic convolutional networks for predicting protein–ligand binding affinity. arXiv preprint arXiv: 170310603
Stepniewska-Dziubinska MM, Zielenkiewicz P, Siedlecki P (2018) Development and evaluation of a deep learning model for protein–ligand binding affinity prediction. Bioinformatics. https://doi.org/10.1093/bioinformatics/bty374
Bengio Y, Vincent AC,P (2013) Representation learning: a review and new perspectives. IEEE Trans Pattern Anal Mach Intell 35(8):31
Meng ECSB, Kuntz ID (1992) Automated docking with grid-based energy evaluation. J Comput Chem 13:20
Jones G, Willett P, Glen RC, Leach AR, Taylor R (1997) Development and validation of a genetic algorithm for flexible docking. J Mol Biol 267(3):727–748. https://doi.org/10.1006/jmbi.1996.0897
Krammer A, Kirchhoff PD, Jiang X, Venkatachalam CM, Waldman M (2005) LigScore: a novel scoring function for predicting binding affinities. J Mol Graph Model 23(5):395–407. https://doi.org/10.1016/j.jmgm.2004.11.007
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47(7):1739–1749. https://doi.org/10.1021/jm0306430
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (2015) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19(14):1639–1662
Steinmann C, Olsson MA, Ryde U (2018) Relative ligand-binding free energies calculated from multiple short QM/MM MD simulations. J Chem Theory Comput 14(6):3228–3237. https://doi.org/10.1021/acs.jctc.8b00081
Chaskar P, Zoete V, Röhrig UF (2014) Toward on-the-fly quantum mechanical/molecular mechanical (QM/MM) docking: development and benchmark of a scoring function. J Chem Inf Model 54(11):3137–3152. https://doi.org/10.1021/ci5004152
Terp GE, Johansen BN, Christensen IT, Jorgensen FS (2001) A new concept for multidimensional selection of ligand conformations (MultiSelect) and multidimensional scoring (MultiScore) of protein–ligand binding affinities. J Med Chem 44(14):2333–2343
Betzi S, Suhre K, Chetrit B, Guerlesquin F, Morelli X (2006) GFscore: a general nonlinear consensus scoring function for high-throughput docking. J Chem Inf Model 46(4):1704–1712. https://doi.org/10.1021/ci0600758
Bar-Haim S, Aharon A, Ben-Moshe T, Marantz Y, Senderowitz H (2009) SeleX-CS: a new consensus scoring algorithm for hit discovery and lead optimization. J Chem Inf Model 49(3):623–633. https://doi.org/10.1021/ci800335j
Plewczynski D, Lazniewski M, von Grotthuss M, Rychlewski L, Ginalski K (2011) VoteDock: consensus docking method for prediction of protein–ligand interactions. J Comput Chem 32(4):568–581. https://doi.org/10.1002/jcc.21642
Zhang L, Liu Y, Wang M, Wu Z, Li N, Zhang J, Yang C (2017) EZH2-, CHD4-, and IDH-linked epigenetic perturbation and its association with survival in glioma patients. J Mol Cell Biol 9(6):477–488. https://doi.org/10.1093/jmcb/mjx056
Zhang L, Xiao M, Zhou J, Yu J (2018) Lineage-associated underrepresented permutations (LAUPs) of mammalian genomic sequences based on a Jellyfish-based LAUPs analysis application (JBLA). Bioinformatics 34(21):3624–3630. https://doi.org/10.1093/bioinformatics/bty392
Santos-Martins D, Forli S, Ramos MJ, Olson AJ (2014) AutoDock4(Zn): an improved AutoDock force field for small-molecule docking to zinc metalloproteins. J Chem Inf Model 54(8):2371–2379. https://doi.org/10.1021/ci500209e
Poli G, Jha V, Martinelli A, Supuran CT, Tuccinardi T (2018) Development of a fingerprint-based scoring function for the prediction of the binding mode of carbonic anhydrase II inhibitors. Int J Mol Sci. https://doi.org/10.3390/ijms19071851
Baek M, Shin WH, Chung HW, Seok C (2017) GalaxyDock BP2 score: a hybrid scoring function for accurate protein–ligand docking. J Comput Aided Mol Design 31(7):653–666. https://doi.org/10.1007/s10822-017-0030-9
Shin WH, Kim JK, Kim DS, Seok C (2013) GalaxyDock2: protein–ligand docking using beta-complex and global optimization. J Comput Chem 34(30):2647–2656. https://doi.org/10.1002/jcc.23438
Debroise T, Shakhnovich EI, Cheron N (2017) A hybrid knowledge-based and empirical scoring function for protein–ligand interaction: SMoG2016. J Chem Inf Model 57(3):584–593. https://doi.org/10.1021/acs.jcim.6b00610
Acknowledgements
This study is supported by the National Natural Science Foundation of China (No. 61372138), and National Science and Technology Major Project of China (No. 2018ZX10201002).
Funding
This study is supported by the National Natural Science Foundation of China (No. 61372138), and National Science and Technology Major Project of China (No. 2018ZX10201002).
Author information
Authors and Affiliations
Contributions
Conception and design: LZ; Writing and revision of the manuscript: JL; ALF.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Li, J., Fu, A. & Zhang, L. An Overview of Scoring Functions Used for Protein–Ligand Interactions in Molecular Docking. Interdiscip Sci Comput Life Sci 11, 320–328 (2019). https://doi.org/10.1007/s12539-019-00327-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-019-00327-w