Abstract
Little is known about early coincidental changes in bone and vascular properties, particularly in the context of skeletal anabolism (puberty) versus relative equilibrium (young adulthood). We aimed to determine if subclinical markers of vascular function were associated with bone mineral content (BMC) and to evaluate the contribution of systemic factors in healthy females ages 14–42 years. Endothelial function was assessed by flow mediated dilatation (FMD), arterial stiffness by pulse wave velocity (PWV) and augmentation index (AIx), blood pressure (BP) by sphygmomanometer, BMC by DXA, and systemic factors by fasting blood draw. General linear models controlled for age, race and height indicated a positive association between systolic BP (SBP) and BMC independent of systemic factors. When stratified by age using 19 years as a cut-point, there was an inverse relationship between AIx75 in adolescents with insulin (P<0.10) or inflammatory markers (P<0.10) in statistical models. Conversely, there was a positive relationship between BMC and both PWV and AIx75 in young adults (P<0.05). The link between bone and the vasculature may be life stage-dependent. In the context of a less dynamic microenvironment in young adult females, metabolic factors appear to moderate less of an effect of hemodynamic properties on the skeleton relative to adolescents.
Similar content being viewed by others
Introduction
Traditionally, cardiovascular disease (CVD) has been viewed either in the context of metabolic disease or as a distinct entity occurring solely as a consequence of aging. Numerous factors have been identified as contributors to CVD, yet vascular calcification is a key determinant in mortality risk (1). Vascular calcification has recently been associated with osteoporosis. Although undoubtedly progressive in nature, mere attribution of skeletal and vascular disease as age-related artifacts largely overlooks the mechanistic intersection whereby skeletal processes overlap those of the vasculature. Women with low bone density have been shown to be at significantly greater risk of vascular calcification than age-matched controls (2), and low bone density has been associated with CVD risk indicators (e.g., hypertension, stroke, atherosclerosis) (3–6). Accordingly, coincidental suboptimal bone mass and earlier onset vascular calcification represents an emerging concern. Independently, the manifestation of risk factors for future disease progression has been identified as early as adolescence. Identifying connections of vascular and bone phenotype may lend valuable insight into disease progression.
The skeleton and the vasculature are continuously remodeled throughout life for maintenance of integrity and function, although actions differ across the life course. In terms of the skeleton, mineralization is favored over resorption to support bone mass accrual until peak height is reached, resulting in an approximately 40% increase in bone mass throughout adolescence. From the end of the rapid growth stage through early adulthood, deposition and resorption are relatively balanced and bone mass increases approximately 15% under normal conditions. At around 40–50 years of age, resorption outpaces deposition resulting in a progressive loss in both sexes. Calcification of vessels follows an opposing course, such that wall thickness is progressively increased and artery lumen patency is reduced.
In general, the process of vascular calcification occurs in the tunica intima of atherosclerotic plaques, promoting plaque rupture as well as stiffening of the vessel (7). Speculatively, extracellular levels of calcium and the pro-inflammatory milieu act as potent accelerators of this pathological process (7–9), whereby calcium from the unstable bone matrix is liberated from hydroxyapatite into circulation altering cellular signaling and response processes within the vasculature. For example, a number of investigations have demonstrated an increased osteogenic capacity of vascular smooth muscle cells (VSMC) with increased exposure to calcium, with subsequent decreased vascular elasticity, increased arterial stiffness and endothelial dysfunction (10–13). In turn, osteogenic VSMC-induced calcium deposition in the vessels has been associated with decreased values of bone density and greater incidence of coronary events (14). Accumulating evidence suggests that as bone mineral resorption accelerates, the process outpaces bone mineralization and the increased calcium in circulation may impact degree of deposition into the vasculature.
The atherosclerotic process leading to vascular calcification begins in youth, with evidence of subclinical signs of vascular compromise appearing as early as adolescence (12,15–18). Elevated blood pressure and arterial stiffness are becoming increasingly common among adolescents. Impairments in skeletal integrity have also been evidenced in adolescence, with the time encom-passing peak bone mass accrual, the commonly implicated period impacting long-term bone health. The subclinical signs of impaired and/or surrogate measures of vascular calcification, as well as assessment of bone mass, can be conducted non-invasively. Despite similar and overlapping pathways, few investigations have been directed at the bone and vasculature intersection, and even fewer have taken into consideration life stage dependent differences. We aimed to identify whether intermediate end-points of vascular calcification are associated with skeletal parameters during adolescence, a dynamic stage of vascular and skeletal remodeling, and young adulthood, a steady stage primarily involving maintenance processes. Age 19 years was used as the cut-point since the majority of BMC (∼90%) is accrued by the end of adolescence. We hypothesized that in both adolescents and young adults bone mineral content (BMC) would be inversely associated with blood pressure, arterial stiffness and endothelial dysfunction.
Methods
Participants
A total of 87 females (59.3% AA), ages 18–42 years, from two observational cohort studies conducted between January 2010 and September 2011 at University of Alabama at Birmingham (UAB): the VIVID and DIVA studies (Clinical Trial Registration Numbers: NCT 01041547 & NCT 01041365, respectively), were included. Exclusion criteria were self-reported history of diabetes, hypertension, or any other condition known to influence insulin sensitivity or vascular function, such as polycystic ovary syndrome; anti-hypertensive, glucose-controlling, or lipid-lowering medications; current smoking status; lactose intolerance; non-ambulatory; or measured BMI >95 percentile for age and sex according to the Centers for Disease Control and Prevention growth charts for adolescents (19) or >32 kg·m−2 for adults. Only adolescents who were menarchal and in Tanner stage ≥4 for breast and pubic hair development were included in the ≤19 year-old group. We limited the age to 42 years as to correspond with the bone life stage per study objectives. Dietary supplemental use was not reported by any participants. Each protocol was approved by the Institution Review Board for human participants at UAB. Written informed consent (parent consent and subject assent for those less than 19 years of age) was obtained before entry to the study.
Protocol
Participants completed all analyses over the course of two visits, no more than one week apart. For the first visit, participants arrived at the CRU after a 12-hour fast for a blood draw, followed by body composition analyses in the Department of Nutrition Sciences. The second visit consisted of vascular function assessments, in which participants came to the Diabetes Research Training Center's Human Physiology Core Cardiodynamic Laboratory having fasted for more than eight hours.
Cardiovascular parameters
Averaged, seated systolic (SBP) and diastolic (DBP) blood pressure were obtained in the UAB Center of Clinical and Translational Science's Clinical Research Unit with three consecutive readings.
Flow-mediated dilatation (FMD) was used as a parameter of endothelial function, while peak wave velocity (PWV) and augmentation index adjusted to a heart rate of 75 beats per min (AIx75), were used as indicators of arterial stiffness. Radial pulse wave analysis (PWA), carotid-femoral PWV and FMD testing were conducted by a single physician. FMD was measured by brachial ultrasound imaging with a 7.5 MHz linear-array probe (Philips HP Agilent Sonos 5500, Andover, MA) according to standard guidelines (20). Radial PWA for assessment of AIx75 and carotid-femoral PWV were performed using SphygmoCorapplanation tonometry system (AtCor Medical, Sydney, Australia) as previously described (21). However, PWV was only performed in adults ≥19 years as this assessment has only been validated in the adult population.
Serum analysis
Blood samples were obtained after a 12-hour fast, from which 25-hydroxy vitamin D (25OHD) and markers of inflammation [interleukin 6 (IL6), C-reactive protein (CRP), vascular endothelial growth factor (VEGF)] were measured. The serum 25OHD assay was obtained commercially (Quest Diagnostics), using Liquid chromatographytandem mass spectrometry. High-sensitivity enzymelinked immunosorbent assays (ELISA) were used to determine CRP (ALPCO Diagnostics, Windham, NH) and IL6 concentrations (R&D Systems, Minneapolis, MN). Multiarray ELISA (Meso Scale Discovery, Maryland) was used to determine VEGF concentrations.
A mixed meal tolerance test (MMTT) was performed using Carnation Instant Breakfast prepared with whole milk in a dosage of 1.5 kcal·kg−1 of lean body mass
[1.75 gm·kg−1 lean body mass of carbohydrate], followed by repeated blood draws at baseline and at 5, 10, 15, 20, 25, 30, 45, 60, 90, 120, 150, and 180 minutes after the start of meal ingestion. Plasma samples were stored at −80 °C until assay. Glucose was assayed in 10 μL sera using a Sirrus analyzer (Stanbio, Boerne, TX). The mean intra- and inter-assay coefficients of variation (CV) for glucose analysis in the Core Laboratory are 1.28% and 1.53%, respectively. Insulin was assayed by immunofluorescence on a TOSOH AIA-II analyzer (TOSOH Corp., South San Francisco, CA); intra-assay CV of 1.5% and inter-assay CV of 4.4%. For data analysis, postprandial insulin and glucose responses were determined as the area under the curve (AUC) with the use of the trapezoidal method (22).
Body composition and anthropometrics
Body composition [fat mass, lean mass and bone mineral content (BMC)] was assessed using dual energy X-ray absorptiometry (DXA; iDXA, GE-Lunar Radiation Corp., Madison, WI) with participants lying in the supine position with their arms at their sides, and anthropometrics (height and weight) were obtained.
Statistical analyses
Descriptive statistics (mean±standard error) were determined for the overall sample and stratified according to age group (adolescents≤19 years, and young adults 19–42 years). General linear models (GLM) were used to assess the associations of BMC with cardiovascular parameters, with cardiovascular parameters as the dependent variables in individual models. All models were controlled for potential confounders including age, race and height. Independent variables were selected based on univariate analysis and from variables known to be associated with BMC according to our previous studies. In subsequent models, the influence of 25OHD levels, insulin area under the curve (AUC) and inflammatory markers (CRP, IL6, VEGF) were investigated respectively. These relationships were also assessed according to age category. All tests were two-sided and assumed a 5% significance level using SAS software (version 9.3; SAS Institute, Cary, NC). Only participants with full data on all tested variables were included in the analyses.
Results
Table 1 presents the descriptive statistics of the overall sample and according to age category. Participants were 14–42 years (mean age 21.5±0.9 years) with an average BMI of 23.7±0.5 kg·m−2. Trunk fat, DBP, AIx75 and 25OHD were greater in those over 19 years of age (P< 0.05). Insulin AUC concentration was greater in those 14–19 years (P<0.05). There were no age category differences in inflammatory parameters.
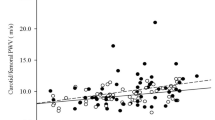
Evaluation of the relationship between bone and subclinical markers of vascular health in the total sample demonstrated a consistent positive association between BMC and SBP (P=0.01, Table 2). This relationship was independent of 25OHD, insulin AUC and markers of inflammation (data not shown). A relationship was not apparent between any measure of vascular health and BMC in the adolescent group, although there was a marginally significant inverse relationship between AIx75 and BMC with inclusion of 25OHD (P=0.10), insulin AUC (P=0.07) and the inflammatory markers, CRP (P=0.08) and VEGF (P=0.10), respectively. In the adult group, the relationship between SBP and BMC retained a marginally significant positive relationship (P=0.10). There was a positive relationship between AIx75 and BMC (P<0.05), and there was a positive association between PWV and BMC in the adult category (P<0.05). These relationships were not influenced by inclusion of 25OHD, insulin AUC, or inflammatory markers (data not shown).
Discussion
Processes contributing to vascular and skeletal maintenance substantially overlap and are highly operational early in the life course. However, linkage has been largely underappreciated outside of the aging population in which CVD and decline in bone integrity have become apparent. While overt disease among the population included in this analysis is rare, it has recently been demonstrated that subclinical markers become apparent as early as puberty (12,15–18). As such, this investigation aimed to identify a link between subclinical markers of vascular function and skeletal phenotype in adolescent and premenopausal females. The findings from this study provide some support for such a connection. We identified a positive association between SBP and BMC. We also observed an age-specific association between arterial stiffness and BMC, such that higher arterial stiffness, as determined as AIx75, and the adult-specific marker, PWV, was positively associated with BMC in adults; conversely, AIx75 was inversely associated with BMC in adolescents. However, endothelial function was not associated with BMC. While associations observed herein are modest, to our knowledge this study represents the first to investigate overlapping vascular and skeletal properties early in life in individuals free of overt disease.
Elevated blood pressure is an early marker of CVD, having a well-established impact on vascular integrity. Infrastructural compromise is initiated via activation of the renin angiotensin system (RAS) by elevated SBP, resulting in increases in cellular adhesion (increased endothelial ‘stickiness’) and macrophage infiltration of the vessel wall (23). In turn, the inflammatory cascade is activated, thereby degrading the endothelial matrix and activating differentiation of vascular smooth muscle cells (VSMC) toward the osteogenic lineage, promoting vascular calcification (24). Beyond the adverse effects of SBP on vascular integrity, speculatively through an inflammatory cascade, SBP has also been associated with increased bone resorption and elevated calcium in circulation (25). Though the mechanisms are not clearly established, two concomitant additive and bidirectional processes are likely involved: 1) vascular calcification may reduce blood perfusion, a critical determinant of adult skeletal formation rate; and 2) increased calcium release into circulation, consequential to age-related bone loss, may promote osteoblast-like differentiation and mineralization of VSMC. Further, it was reported that changes in the peripheral vascular matrix may be associated with cumulative exposure to the adverse effects of SBP (26). Accordingly, we expected to observe an inverse association between SBP and BMC. Rather, we observed a positive association in the total sample, which appeared to be driven by the young adult group, as opposed to those earlier on in development, thus having less exposure to detrimental consequences of elevated SBP. This study population included healthy, normotensive females, likely explaining the counterintuitive reports in postmenopausal women and/or those with previously diagnosed hypertension (27,28). SBP within the normal range may not correspond with the same mechanistic pathways, including RAS activation as seen in clinically hypertensive individuals. Despite no association of SBP with BMC in the adolescent population, it is known that occurrence of SBP in adolescence induces long-term adverse influence on the atherosclerotic process (15,29).
We also observed a positive association between arterial stiffness and BMC in the adult women. As in most regulated biological systems, catabolic and anabolic processes are coupled. As a conduit for delivery of nutrients, changes in the vessel matrix (and consequent release of growth factors, proteins and hormones) serving the skeleton may initiate a compensatory cascade of events, which initially favor bone calcium deposition in young adults. This may be analogous to the maintenance of BMC in obesity and type 2 diabetes (despite increased skeletal fragility), two conditions in which long-term health effects include skeletal and vascular compromise. In that context, prolonged exposure to inflammation and perturbed bone-vascular interactions may have long-term deleterious skeletal effects (12,23).
Among adolescents, a marginal inverse association between AIx75 and BMC was observed. This may reflect the differences in the metabolic state of the adolescents, in which lower 25OHD level and higher insulin level relative to the adults was noted (Table 1). Further, the inflammatory profile was similar to that of the young adults. While not independently associated, inclusion of these variables in statistical models strengthened the inverse association between BMC and AIx75. It is plausible that metabolic perturbances advance accumulation of cardiovascular- and bone-related risk factors, and may underlie increasing morbidity and mortality risk among youth (17).
To our knowledge, no study has assessed these relationships, particularly among a young population with comprehensive phenotyping of subclinical markers of vascular function presented herein. Due to availability and cost, studies which investigate intermediate endpoints of vascular disease, such as endothelial dysfunction, as determined by FMD, and arterial stiffness, as determined by PWV and AIx75, are few. The study, however, is not without limitations. The small sample size combined with the methodological approach to phenotype bone may not have allowed for the detection of significant relationships explored. Evaluation of the strength-structure relationship requires consideration of both outer and inner surfaces, and an assessment of markers indicative of bone turnover. Although BMC gives a quantitative aspect of bone, it does not capture structural properties. Speculatively, the link between subclinical markers of CVD and bone integrity would involve mineral resorption, an action which prominently occurs on the inner surface of bone at the endosteum. Further studies are required in order to characterize the life stage-dependency of bone-vessel interplay.
Coexistence of impairments in bone and vascular integrity is largely attributed to vessel wall calcification, speculatively induced by skeletal calcium loss being deposited in the vessel wall. Although this study did not investigate the specific mechanisms, support for overlapping subclinical signs of compromise emerged. These data add evidence for a life stage-dependent link between cardiovascular parameters and the skeleton. Vasculature function, early in life when skeletal integrity is susceptible to microenvironmental changes, could have profound effects later in life. Moreover, in the context of less dynamic bone remodeling as detected in healthy pre-menopausal adult females, metabolic factors (vitamin D status, insulin homeostasis and inflammatory processes) appear to moderate less of an effect of hemodynamic properties on the skeleton relative to adolescents.
References
Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, De Leeuw PW, Kroon AA . Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5:185–197.
von der RP, Hansen MA, Hassager C . The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106:273–278.
Danilevicius CF, Lopes JB, Pereira RM . Bone metabolism and vascular calcification. Braz J Med Biol Res. 2007;40:435–442.
Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW . Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276.
Sinnott B, Syed I, Sevrukov A, Barengolts E . Coronary calcification and osteoporosis in men and postmenopausal women are independent processes associated with aging. Calcif Tissue Int. 2006;78:195–202.
Stojanovic OI, Lazovic M, Lazovic M, Vuceljic M . Association between atherosclerosis and osteoporosis, the role of vitamin D. Arch Med Sci. 2011;7:179–188.
Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM . Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011;109:697–711.
New SE, Aikawa E . Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res. 2011;108:1381–1391.
Shroff RC, McNair R, Skepper JN, Figg N, Schurgers LJ, Deanfield J, Rees L, Shanahan CM . Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol. 2010;21:103–112.
Karwowski W, Naumnik B, Szczepanski M, Mysliwiec M . The mechanism of vascular calcification — a systematic review. Med Sci Monit. 2012;18:RA1–RA11.
Nakagami H, Osako MK, Morishita R . New concept of vascular calcification and metabolism. Curr Vasc Pharmacol. 2011;9:124–127.
Thompson B, Towler DA . Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol. 2012;8:529–543.
Yang X, Cai X, Wang J, Tang H, Yuan Q, Gong P, Lin Y . Mechanical stretch inhibits adipogenesis and stimulates osteogenesis of adipose stem cells. Cell Prolif. 2012;45:158–166.
Bagger YZ, Rasmussen HB, Alexandersen P, Werge T, Christiansen C, Tanko LB . Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int. 2007;18:505–512.
Aatola H, Magnussen CG, Koivistoinen T, Hutri-Kahonen N, Juonala M, Viikari JS, Lehtimaki T, Raitakari OT, Kahonen M . Simplified Definitions of Elevated Pediatric Blood Pressure and High Adult Arterial Stiffness. Pediatrics. 2013;132:e70–e76.
Pierce GL, Zhu H, Darracott K, Edet I, Bhagatwala J, Huang Y, Dong Y . Arterial stiffness and pulse-pressure amplification in overweight/obese African-American adolescents: relation with higher systolic and pulse pressure. Am J Hypertens. 2013;26:20–26.
Saydah S, Bullard KM, Imperatore G, Geiss L, Gregg EW . Cardiometabolic risk factors among US adolescents and young adults and risk of early mortality. Pediatrics. 2013;131:e679–e686.
Wadwa RP, Urbina EM, Anderson AM, Hamman RF, Dolan LM, Rodriguez BL, Daniels SR, Dabelea D . Measures of arterial stiffness in youth with type 1 and type 2 diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2010;33:881–886.
Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL . CDC growth charts: United States. Adv Data. 2000;(314):1–27.
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265.
Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell'Italia LJ, Calhoun DA . Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54:475–481.
Matthews JN, Altman DG, Campbell MJ, Royston P . Analysis of serial measurements in medical research. BMJ. 1990;300:230–235.
Castellon X, Bogdanova V . Screening for subclinical atherosclerosis by noninvasive methods in asymptomatic patients with risk factors. Clin Interv Aging. 2013;8:573–580.
Osako MK, Nakagami H, Shimamura M, Koriyama H, Nakagami F, Shimizu H, Miyake T, Yoshizumi M, Rakugi H, Morishita R . Cross-Talk of Receptor Activator of Nuclear Factor-kappaB Ligand Signaling With Renin-Angiotensin System in Vascular Calcification. Arterioscler Thromb Vasc Biol. 2013;33:1287–1296.
Cappuccio FP . The “calcium antihypertension theory”. Am J Hypertens. 1999;12:93–95.
Murgan I, Beyer S, Kotliar KE, Weber L, Bechtold-Dalla PS, Dalla PR, Wegner A, Sitnikova D, Stock K, Heemann U, Schmaderer C, Baumann M . Arterial and retinal vascular changes in hypertensive and prehypertensive adolescents. Am J Hypertens. 2013;26:400–408.
Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA . High blood pressure and bone-mineral loss in elderly white women: a prospective study. Study of Osteoporotic Fractures Research Group. Lancet. 1999;354:971–975.
Tsuda K, Nishio I, Masuyama Y . Bone mineral density in women with essential hypertension. Am J Hypertens. 2001;14:704–707.
Juhola J, Magnussen CG, Viikari JS, Kahonen M, Hutri-Kahonen N, Jula A, Lehtimaki T, Akerblom HK, Pietikainen M, Laitinen T, Jokinen E, Taittonen L, Raitakari OT, Juonala M . Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159:584–590.
Acknowledgements
The authors thank the investigators, staff, and participants of the clinical trials for their valuable contributions. This study included clinical trials, NCT010141365 and NCT 01041547. PI's, AA was supported in part by Child health Research Center Grant K12 HD043397 (T0909180013) and JAA was supported by the American Heart Association (Greater Southeast Affiliate) and was funded by UAB Diabetes Research Training Center (P60 DK079626) and supported by the Center for Clinical and Translational Science (5UL1 RR025777). LJH was supported by T32DK007545.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanks, L., Ashraf, A., Gower, B. et al. Subclinical Indication of Linkage Between Markers of Skeletal and Cardiovascular Properties. Bone Res 1, 291–297 (2013). https://doi.org/10.4248/BR201303007
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.4248/BR201303007