Abstract
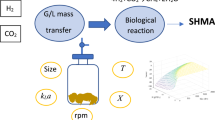
Gas-to-liquid mass transfer of hydrogen (H2) was investigated in a gas–liquid reactor with a continuous gas phase, a batch liquid phase, and liquid mixing regimes relevant to assessing kinetics of microbial H2 consumption. H2 transfer was quantified in real-time with a H2 microsensor for no mixing, moderate mixing [100 rotations per minute (rpm)], and rapid mixing (200 rpm). The experimental results were simulated by mathematical models to find best-fit values of volumetric mass transfer coefficients—kLa—for H2, which were 1.6/day for no mixing, 7/day for 100 rpm, and 30/day for 200 rpm. Microbiological H2-consumption experiments were conducted with Methanobacterium bryantii M.o.H. to assess effects of H2 mass transfer on microbiological H2-threshold studies. The results illustrate that slow mixing reduced the gas-to-liquid H2 transfer rate, which fell behind the rate of microbiological H2 consumption in the liquid phase. As a result, the liquid-phase H2 concentration remained much lower than the liquid-phase H2 concentration that would be in equilibrium with the gas-phase H2 concentration. Direct measurements of the liquid-phase H2 concentration by an in situ probe demonstrated the problems associated with slow H2 transfer in past H2 threshold studies. The findings indicate that some of the previously reported H2-thresholds most likely were over-estimates due to slow gas-to-liquid H2 transfer. Essential requirements to conduct microbiological H2 threshold experiments are to have vigorous mixing, large gas-to-liquid volume, large interfacial area, and low initial biomass concentration.




Similar content being viewed by others
References
Aulenta F, Beccari M, Majone M, Papini MP, Tandoi V (2008) Competition for H2 between sulfate reduction and dechlorination in butyrate-fed anaerobic cultures. Process Biochem 43:161–168
Boltz JP, Smets BF, Rittmann BE et al (2017) From biofilm ecology to reactors: a focused review. Wat Sci Technol 75(8):1753–1760
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 78:248–254
Brock TD, Brock ML (1968) Measurement of steady-state growth rates of a thermophilic alga directly in nature. J Bacteriol 95(3):811–815
Chapelle FH, Haack SK, Adriaens P, Henry MA, Bradley PM (1996) Comparison of Eh and H2 measurements for delineating redox processes in a contaminated aquifer. Environ Sci Technol 30(12):3565–3569
Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO2, CH4, OCS, N2O, and NO). Microbiol Mol Biol Rev 60(4):609–640
Conrad R, Wetter B (1990) Influence of temperature on energetics of hydrogen metabolism in homoacetogenic, methanogenic, and other anaerobic bacteria. Arch Microbiol 155(1):94–98
Cord-Ruwisch R, Seitz H-J, Conrad R (1988) The capacity of hydrogenotrophic anaerobic bacteria to compete for traces of hydrogen depends on the redox potential of the terminal electron acceptor. Archv Microbiol 149(4):350–357
Cord-Ruwisch R, Mercz TI, Hoh C-Y, Strong GE (1997) Dissolved hydrogen concentration as an on-line parameter for the automated operation and optimization of anaerobic digesters. Biotech Bioeng 56(6):626–634
Faybishenko B, Hazen TC, Long PE, Brodie EL et al (2008) In situ long-term reductive bioimmobilization of Cr(VI) in groundwater using hydrogen release compound. Env Sci Technol 42(22):8478–8585
Fennell DE, Gossett JM, Zinder SH (1997) Comparison of butyric acid, ethanol, lactic acid, and propionic acid as hydrogen donors for the reductive dechlorination of tetrachloroethene. Environ Sci Technol 31:918–926
Heimann AC, Friis AK, Scheutz C, Jakobsen R (2007a) Dynamics of reductive TCE dechlorination in two distinct H2 supply scenarios and at various temperatures. Biodegradation 18:167–179
Heimann AC, Blodau C, Postma D, Larsen F, Viet PH, Nhan PQ (2007b) H2-thresholds and steady-state concentrations associated with microbial arsenate respiration. Environ Sci Technol 44(7):24–33
Heimann A, Jakobsen R, Blodau C (2010) Energetic constrains on H2-Dependent terminal electron accepting processes in anoxic environments: a review of observations and model approaches. Environ Sci Technol 44(1):24–33
Hoehler TM, Alperin MJ, Albert DB, Martens CS (1998) Thermodynamic control on hydrogen concentrations in anoxic sediments. Geochim Cosmochim Acta 62(10):1745–1756
Jakobsen R, Albrechtsen HJ, Rasmussen M, Bay H, Bjerg PL, Christensen TH (1998) H2 concentrations in a landfill leachate plume (Grindsted, Denmark): in situ energetics of terminal electron acceptor processes. Environ Sci Technol 32:2142–2148
Jud G, Schneider K, Bachofen R (1997) The role of hydrogen mass transfer for the growth kinetics of Methanobacterium thermoautotrophicum in batch and chemostat cultures. J Ind Microbiol Biotech 19(4):246–251
Karadagli F, Rittmann BE (2005) Kinetic Characterization of Methanobacterium bryantii M.o.H. Environ Sci Technol 39(13):4900–4905
Karadagli F, Rittmann BE (2007a) Thermodynamic and kinetic analysis of the H2 threshold for Methanobacterium bryantii M.o.H. Biodegradation 18(4):439–452
Karadagli F, Rittmann BE (2007b) A mathematical model for the kinetics of Methanobacterium bryantii M.o.H. considering H2.thresholds. Biodegradation 18(4):453–464
Laiby P, Jakobsen R, Smolders E, Albrecthsen H-J, Bjerg PL (2016) Reductive dechlorination of trichloroethylene (TCE) in competition with Fe and Mn oxides—observed dynamics in H2-dependent terminal electron accepting processes. Geomicrobiology J 33(5):357–366
Löffler FE, Tiedje JM, Sanford RA (1999) Fraction of electrons consumed in electron acceptor reduction and hydrogen threshold as indicators of halorespiratory physiology. Appl Environ Microbiol 65:1373–1379
Lovley DR (1985) Minimum threshold for hydrogen metabolism in methanogenic bacteria. Appl Environ Microbiol 49(6):1530–1531
Lovley DR, Goodwin ST (1988) Hydrogen concentrations as an indicator of the predominant terminal electron-accepting reactions in aquatic sediments. Geochim Cosmochim Acta 52(12):2993–3003
Lovley DR, Dwyer DF, Klug MJ (1982) Kinetic analysis of competition between sulfate reducers and methanogens for hydrogen in sediments. Appl Environ Microbiol 43(6):1373–1379
Lovley DR, Greening RC, Ferry JG (1984) Rapidly growing rumen methonogenic microorganism that synthesize coenzyme M and has a affinity for formate. Appl Environ Microbiol 48(1):81–87
Lovley DR, Chapelle FH, Woodward JC (1994) Use of dissolved H2 concentrations to determine distribution of microbially catalyzed redox reactions in anoxic groundwater. Environ Sci Tech 28(7):1205–1210
Lu XX, Tao S, Bosma T, Gerritse J (2001) Characteristic hydrogen concentrations for various redox processes in batch study. J Environ Sci Health Part A 36:1725–1734
Luijten MLGC, Roelofsen W, Langenhoff AAM, Schraa G, Stams AJM (2004) Hydrogen threshold concentrations in pure cultures of halorespiring bacteria and at site polluted with chlorinated ethenes. Environ Microbiol 6:646–650
Nerenberg R (2016) The membrane-biofilm reactor (MBfR) as a counte-diffusional biofilm process. Curr Opin Biotechnol 38:131–136
Pauss A, Andre G, Perrier M, Guiot SR (1990a) Liquid-to-gas mass transfer in anaerobic processes: inevitable transfer limitations of methane and hydrogen in the biomethanation process. App Env Microbiol 56(6):1636–1644
Pauss A, Samson PR, Guiot S, Beauchemin C (1990b) Continuous measurement of dissolved H2 in an anaerobic reactor using a new hydrogen/air fuel cell reactor. Biotech Bioeng 35(5):492–501
Rittmann BE (2018) Biofilms, active substrata, and me. Water Res 132:135–145
Strong GE, Cord-Ruwisch R (1995) An in situ dissolved hydrogen probe for monitoring anaerobic digesters under overload conditions. Biotech Bioeng 45(1):63–68
Thauer RK, Kaster A-K, Seedorf A, Buckel W, Hedderich R (2008) Methanogenic archea: ecologically relevant differences in energy conversation. Nat Rev Microbiol 6(8):579–591
Wilhelm E, Battino R, Wilcock RJ (1977) Low-pressure solubility of gases in liquid water. Chem Rev 77(2):219–262
Yang Y, McCarty PL (1998) Competition for hydrogen within a chlorinated solvent dehalogenating anaerobic mixed culture. Environ Sci Technol 32(22):3591–3597
Acknowledgements
We thank the Instrument and Prototype Machine Shop of Arizona State University for their help with design and manufacturing of the closure system for the test reactor. We thank Drs. Cesar Torres and Yen-jung Lai for their help with the experimental part of this work. We thank Drs. Ferran-Garcia Pitchel and Juan Maldonado Ortiz for providing the H2-sensor system. This work was supported by the Scientific and Technological Council of Turkey (TUBITAK) 2219 – BIDEB – International Post-doctoral Fellowship Programme; and the Swette Trust, Phoenix, Arizona, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of ınterest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karadagli, F., Marcus, A.K. & Rittmann, B.E. Role of hydrogen (H2) mass transfer in microbiological H2-threshold studies. Biodegradation 30, 113–125 (2019). https://doi.org/10.1007/s10532-019-09870-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-019-09870-1