Abstract
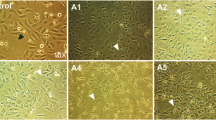
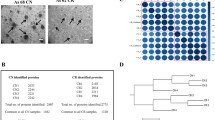
Ovarian cancer is considered to be the most lethal type of gynecological cancer. During the advanced stages of ovarian cancer, an accumulation of ascites is observed. Fucosylation has been classified as an abnormal post-translational modification that is present in many diseases, including ovarian cancer. Ovarian cancer cells that are cultured with ascites stimulation change their morphology; concomitantly, the fucosylation process is altered. However, it is not known which fucosylated proteins are modified. The goal of this work was to identify the differentially fucosylated proteins that are expressed by ovarian cancer cell lines that are cultured with ovarian cancer patients’ ascites. Aleuria aurantia lectin was used to detect fucosylation, and some changes were observed, especially in the cell membrane. Affinity chromatography and mass spectrometry (MALDI-TOF) were used to identify 6 fucosylated proteins. Four proteins (Intermediate filament family orphan 1 [IFFO1], PHD finger protein 20-like protein 1 [PHF20L1], immunoglobulin gamma 1 heavy chain variable region partial [IGHV1–2], and Zinc finger protein 224 [ZNF224]) were obtained from cell cultures stimulated with ascites, and the other two proteins (Peregrin [BRPF1] and Dystrobrevin alpha [DTNA]) were obtained under normal culture conditions. The fucosylated state of some of these proteins was further analyzed. The experimental results show that the ascites of ovarian cancer patients modulated the fucosylation process. The PHD finger protein 20-like protein 1, Zinc finger protein 224 and Peregrin proteins colocalize with fucosylation at different levels.





Similar content being viewed by others
References
GLOBOCAN, 2018. Estimated cancer incidence and prevalence woldwide in 2018. [online] Available at: http://globocan.iarc.fr [Last access: 24th March 2019]
Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC (2001) Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev 22(2):255–288
Cvetkovic D (2003) Early events in ovarian oncogenesis. Reprod Biol Endocrinol 1(68):68. https://doi.org/10.1186/1477-7827-1-68
Odicino F, Pecorelli S, Zigliani L, Creasman W (2008) History of the FIGO cancer staging system. Int J Gynecol Obstet 101(2):205–210. https://doi.org/10.1016/j.ijgo.2007.11.004
Pasquet M, Golzio M, Mery E, Rafii A, Benabbou N, Mirshahi P, Hennebelle I, Bourin P, Allal B, Teissie J, Mirshahi M, Couderc B (2010) Hospicells (ascites-derived stromal cells) promote tumorigenicity and angiogenesis. Int J Cancer 126(9):2090–2101. https://doi.org/10.1002/ijc.24886
Lane D, Bachvarov D, Rancourt C, Piche A (2014) Role of malignant ascites on human mesothelial cells and their gene expression profiles. BMC Cancer 14:288. https://doi.org/10.1186/1471-2407-14-288
Shender VO, Pavlyukov MS, Ziganshin RH, Arapidi GP, Kovalchuk SI, Anikanov NA, Altukhov IA, Alexeev DG, Butenko IO, Shavarda AL, Khomyakova EB, Evtushenko E, Ashrafyan LA, Antonova IB, Kuznetcov IN, Gorbachev AY, Shakhparonov MI, Govorun VM (2014) Proteome–metabolome profiling of ovarian Cancer ascites reveals novel components involved in intercellular communication. Mol Cell Proteomics 13(12):3558–3571. https://doi.org/10.1074/mcp.M114.041194
Bery A, Leung F, Smith CR, Diamandis EP, Kulasingam V (2014) Deciphering the ovarian cancer ascites fluid peptidome. Clin Proteomics 11(1):13. https://doi.org/10.1186/1559-0275-11-13
Pinho S, Reis C (2015) Glycosylation in cancer: mechanism and clinical implications. Nat Rev Cancer 15(9):540–555. https://doi.org/10.1016/j.neo.2018.06.001
Miyoshi E, Moriwaki K, Nakagawa T (2008) Biological function of fucosylation in cancer biology. J Biochem 143(6):725–729. https://doi.org/10.1093/jb/mvn011
Shah M, Telang S, Shah P, Patel P (2008a) Tissue and serum α2-3-and α2-6-linkage specific sialylation changes in oral carcinogenesis. Glycoconj J 25(3):279–290. https://doi.org/10.1007/s10719-007-9086-4
Shah M, Telang S, Raval G, Shah P, Patel PS (2008b) Serum fucosylation changes in oral cancer and oral precancerous conditions. Cancer J 113(2):336–346. https://doi.org/10.1002/cncr.23556
Vajaria B, Patel P (2016) Glycosylation: a halmark of cancer? Glycoconj J 34(2):147–156. https://doi.org/10.1007/s10719-016-9755-2
Hu Z, Cai M, Deng L, Zhu L, Gao J, Tan M, Liu J, Lin B (2016) The fucosylated CD147 enhances the autophagy in epithelial ovarian cancer cells. Oncotarget 7(50):82921–82932. https://doi.org/10.18632/oncotarget.13289
Zhuang H, Hu Z, Tan M, Zhu L, Liu J, Liu D, Yan L, Lin B (2014) Overexpression of Lewis y antigen promotes human epididymis protein 4-mediated invasion and metastasis of ovarian cancer cells. Biochimie 105:91–98. https://doi.org/10.1016/j.biochi.2014.06.022
Cai M, Jin S, Deng L, Zhu L, Hu Z, Liu D, Liu J, Tan M, Gao J, Wang H, Lin B (2017) Lewis y antigen promotes p27 degradation by regulating ubiquitin-proteasome activity. Oncotarget 8(66):110064–110076. https://doi.org/10.18632/oncotarget.22617
Liu J, Zheng M, Qi Y, Wang H, Liu M, Liu Q, Lin B (2018) Lewis(y) antigen-mediated positive feedback loop induces and promotes chemotherapeutic resistance in ovarian cancer. Int J Oncol 53(4):1774–1786. https://doi.org/10.3892/ijo.2018.4496
Thomson S, Dargan E, Turner GA (1992) Increased fucosylation and other carbohydrate changes in haptoglobin in ovarian cancer. Cancer Lett 66:43–48
Garibay-Cerdenares OL, Hernández-Ramírez VI, Osorio-Trujillo JC, Hernández-Ortíz M, Gallardo-Rincón D, Cantú de León D, Encarnación-Guevara S, Villegas-Pineda JC, Talamás-Rohana P (2014) Proteomic identification of fucosylated haptoglobin alpha isoforms in ascitic fluids and its localization in ovarian carcinoma tissues from Mexican patients. J Ovarian Res 7:27. https://doi.org/10.1186/1757-2215-7-27
Villegas-Pineda JC, Garibay-Cerdenares OL, Hernández-Ramírez VI, Gallardo-Rincón D, Cantú de León D, Pérez-Montiel-Gómez MD, Talamás-Rohana P (2015) Integrins and haptoglobin: molecules overexpressed in ovarian cancer. Pathol Res Pract 211(12):973–981. https://doi.org/10.1016/j.prp.2015.10.002
Yarnashita K, Korde N, Endo T, Iwaki Y, Kobata A (1989) Altered glycosylation of serum transferrin of patients with hepatocellular carcinoma. J Biol Chem 264:2415–2423
Aoyagi Y, Isernura M, Yosizawa Z, Suzuki Y, Sekine C, Ono T, Ichida F (1985) Fucosylation of serum o-fetoprotein in patients with primary hepatocellular carcinoma. Biochim Biophs Acta 830:217–223
Yamamoto K, Tsujii T, Tarutani O, Osawa T (1984) Structural changes of carbohydrate chains of human thyroglobulin accompanying tranformations of thyroid glands. Eur J Biochem 143:133–144
Toledo-Leyva A, Villegas-Pineda JC, Encarnación-Guevara S, Gallardo-Rincón D, Talamás Rohana P (2018) Effect of ovarian cancer ascites on SKOV-3 cells proteome: new proteins associated with aggressive phenotype in epithelial ovarian cancer. Proteome Sci 16:3. https://doi.org/10.1186/s12953-018-0133-9
Carduner L, Picot CR, Leroy-Dudal J, Blay L, Kellouche S, Carreiras F (2014) Cell cycle arrest or survival signaling through αv integrins, activation of PKC and ERK1/2 lead to anoikis resistance of ovarian cancer spheroids. Exp Cell Res 320(2):329–342. https://doi.org/10.1016/j.yexcr.2013.11.011
Runyon BA (1994) Care of patients with ascites. N Engl J Med 330:337–342. https://doi.org/10.1056/NEJM199402033300508
Shen-Gunther J, Mannel RS (2002) Ascites as a predictor of ovarian malignancy. Gynecol Oncol 87(1):77–83
Offner FA, Obrist P, Stadlmann S, Feichtinger H, Klingler P, Herold M, Zwierzina H, Hittmair A, Mikuz G, Abendstein B, Zeimet A, Marth C (1995) IL-6 secretion by human peritoneal mesothelial and ovarian cancer cells. Cytokine 7(6):542–547
Matte I, Lane D, Laplante C, Rancourt C, Piche A (2012) Profiling of cytokines in human epithelial ovarian cancer ascites. Am J Cancer Res 2(5):566–580
Matte I, Lane D, Bachvarov D, Rancourt C, Piche A (2014) Role of malignant ascites on human mesothelial cells and their gene expression profiles. BMC Cancer 14:288. https://doi.org/10.1186/1471-2407-14-288
Matte I, Lane D, Laplante C, Garde-Granger P, Rancourt C, Piche A (2015) Ovarian cancer ascites enhance the migration of patient-derived peritoneal mesothelial cells via cMet pathway through HGF-dependent and -independent mechanisms. Int J Cancer 137(2):289–298. https://doi.org/10.1002/ijc.29385
Bhowmick NA, Neilson EG, Moses HL (2004) Stromal fibroblasts in cancer initiation and progression. Nature 432(7015):332–337
Wels J, Kaplan RN, Rafii S, Lyden D (2008) Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev 22(5):559–574. https://doi.org/10.1101/gad.1636908
Villegas-Pineda JC, Toledo-Leyva A, Osorio-Trujillo C, Hernández-Ramírez VI, Talamás-Rohana P (2017) The translational blocking of α5 and α6 integrin subunits affects migration and invasion, and increases sensitivity to carboplatin of SKOV-3 ovarian cancer cell line. Exp Cell Res 351(2):127–134. https://doi.org/10.1016/j.yexcr.2017.01.010
So KA, Min KJ, Hong JH, Lee JK (2015) Interleukin-6 expression by interactions between gynecologic cancer cells and human mesenchymal stem cells promotes epithelial–mesenchymal transition. Int J Oncol 47(4):1451–1459. https://doi.org/10.3892/ijo.2015.3122
Lane D, Matte I, Rancourt C, Piche A (2012) Osteoprotegerin (OPG) protects ovarian cancer cells from TRAIL-induced apoptosis but does not contribute to malignant ascites-mediated attenuation of TRAIL-induced apoptosis. J Ovarian Res 5(1):34. https://doi.org/10.1186/1757-2215-5-34
Reid PE, Brown NJ, Holen I (2009) Breast cancer cells stimulate osteoprotegerin (OPG) production by endothelial cells through direct cell contact. Mol Cancer 8:49. https://doi.org/10.1186/1476-4598-8-49
Yin J, Zeng F, Wu N, Kang K, Yang Z, Yang H (2015) Interleukin-8 promotes human ovarian cancer cell migration by epithelial-mesenchymal transition induction in vitro. Clin Transl Oncol 17(5):365–370. https://doi.org/10.1007/s12094-014-1240-4
Lane D1, Matte I, Rancourt C, Piché A (2011) Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer 30(11):210. https://doi.org/10.1186/1471-2407-11-210
Giuntoli RL, Webb TJ, Zoso A, Rogers O, Diaz-Montes TP, Bristow RE, Oelke M (2009) Ovarian cancer-associated ascites demonstrates altered immune environment: implications for antitumor immunity. Anticancer Res 29:2875–2884
Terao N, Takamatsu S, Minehira T, Sobajima T, Nakayama K, Kamada Y, Miyoshi E (2015) Fucosylation is a common glycosylation type in pancreatic cancer stem cell-like phenotypes. World J Gastroenterol 21(13):3876–3887. https://doi.org/10.3748/wjg.v21.i13.3876
Syed V, Ulinski G, Mok SC, Ho SM (2002) Reproductive hormone-induced, STAT3-mediated interleukin 6 action in normal and malignant human ovarian surface epithelial cells. J Natl Cancer Inst 94:617–629
Obata NH, Tamakoshi K, Shibata K, Kikkawa F, Tomoda Y (1997) Effects of interleukin-6 on in vitro cell attachment, migration and invasion of human ovarian carcinoma. Anticancer Res 17:337–342
Nilsson MB, Langley RR, Fidler IJ (2005) Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res 65:10794–10800. https://doi.org/10.1158/0008-5472.CAN-05-0623
Wang Y, Xu RC, Zhang XL, Niu XL, Qu Y, Li LZ, Meng XY (2012) Interleukin-8 secretion by ovarian cancer cells increases anchorage-independent growth, proliferation, angiogenic potential, adhesion and invasion. Cytokine 59(1):145–155. https://doi.org/10.1016/j.cyto.2012.04.013
Soon H, Moon A (2010) Epithelial-mesenchymal transition and cell invasion. Toxicol Res 26(4):245–252. https://doi.org/10.5487/TR.2010.26.4.245
Smith PL, Myers JT, Rogers CE, Zhou L, Petryniak B, Becker DJ, Homeister JW, Lowe JB (2002) Conditional control of selectin ligand expression and global fucosylation events in mice with a targeted mutation at the FX locus. J Cell Biol 158:801–815
Chao YB, Scovell WM, Yan SB (1994) High mobility group protein, HMG-1, contains insignificant glycosyl modification. Protein Sci 3(12):2452–2454. https://doi.org/10.1002/pro.5560031230
Hart GW, Haltiwanger RS, Holt GD, Kelly WG (1989) Glycosylation in the nucleus and cytoplasm. Annu Rev Biochem 58:841–874
Feizi T (2000) Carbohydrate-mediated recognition systems in innate immunity. Immunol Rev 173:79–88
Marth JD, Grewal PK (2008) Mammalian glycosylation in immunity. Nat Rev Immunol 8:874–887
Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV, Bogatyrev SR, Ismagilov RF, Pamer EG, Turnbaugh PJ, Chervonsky AV (2014) Rapid fucosylation of intestinal epithelium sustains host–commensal symbiosis in sickness. Nature 514:638–641. https://doi.org/10.1038/nature13823
Myers J, Huang Y, Wei L, Yan Q, Huang A, Zhou L (2010) Fucose-deficient hematopoietic stem cells have decreased self-renewal and aberrant marrow niche occupancy. Transfusion 50:2660–2669. https://doi.org/10.1111/j.1537-2995.2010.02745.x
Soochi K, Boyun K, Sang Song Y (2016) Ascites modulates cancer cell behavior, contributing to tumor heterogeneity in ovarian cancer. Cancer Sci 107(9):1173–1178. https://doi.org/10.1111/cas.12987
Larsen RD, Ernst LK, Nair RP, Lowe JB (1990) Molecular cloning, sequence, and expression of a human GDP-L-fucose:beta-D-galactoside 2-alpha-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc Natl Acad Sci U S A 87:6674–6678
Kelly RJ, Ernst LK, Larsen RD, Bryant JG, Robinson JS, Lowe JB (1994) Molecular basis for H blood group deficiency in Bombay (oh) and Para-Bombay individuals. Proc Natl Acad Sci U S A 91:5843–5847
Hooper LV, Gordon JI (2001) Glycans as legislators of host-microbial interactions: spanning the spectrum from symbiosis to pathogenicity. Glycobiol 11:1R–10R. https://doi.org/10.1093/glycob/11.2.1R
Tonetti M, Sturla L, Bisso A, Benatti U, De Flora A (1996) Synthesis of GDP-L fucose by the human FX protein. J Biol Chem 271(44):27274–27279
Lowe JB (1997) Selectin ligands, leukocyte trafficking, and fucosyltransferase genes. Kidney Int 51(5):1418–1426
Wang Y, Shao L, Shi S, Harris RJ, Spellman MW, Stanley P, Haltiwanger RS (2001) Modification of epidermal growth factor-like repeats with O-fucose. Molecular cloning and expression of a novel GDP-fucose protein O-fucosyltransferase. J Biol Chem 276(43):40338–40345
Feng X, Zhao L, Gao S, Song X, Dong W, Zhao Y, Zhou H, Cheng L, Miao X, Jia L (2016) Increased fucosylation has a pivotal role in multidrug resistance of breast cancer cells through miR-224-3p targeting FUT4. Gene 578(2):232–241. https://doi.org/10.1016/j.gene.2015.12.028
Carrascal MA, Silva M, Ramalho JS, Pen C, Martins M, Pascoal C, Amaral C, Serrano I, Oliveira MJ, Sackstein R, Videira PA (2017) Inhibition of fucosylation in human invasive ductal carcinoma reduces E-selectin ligand expression, cell proliferation and ERK1/2 and p38 MAPK activation. Mol Oncol 2(5):579–593. https://doi.org/10.1002/1878-0261.12163
Zhou Y, Fukuda T, Hang Q, Hou S, Isaji T, Kameyama A, Gu J (2017) Inhibition of fucosylation by 2-fluorofucose suppresses human liver cancer HepG2 cell proliferation and migration as well as tumor formation. Sci Rep 7(1):11563. https://doi.org/10.1038/s41598-017-11911-9
Ohata S, Kinoshita S, Aoki R, Tanaka H, Wada H, Tsuruoka-Kinoshita S, Tsuboi T, Watabe S, Okamoto H (2009) Neuroepithelial cells require fucosylated glycans to guide the migration of vagus motor neuron progenitors in the developing zebrafish hindbrain. Development 136(10):1653–1663. https://doi.org/10.1242/dev.033290
Moriwaki K, Narisada M, Imai T, Shinzaki S, Miyoshi E (2010) The effect of epigenetic regulation of fucosylation on TRAIL-induced apoptosis. Glycoconj J 27(7–9):649–659. https://doi.org/10.1007/s10719-010-9310-5
Blanas A, Sahasrabudhe NM, Rodríguez E, van Kooyk Y, van Vliet SJ (2018) Fucosylated antigens in Cancer: an Alliance toward tumor progression, metastasis, and resistance to chemotherapy. Front Oncol 23(8):39. https://doi.org/10.3389/fonc.2018.00039
Lv X, Song J, Xue K, Li Z, Li M, Zahid D, Cao H, Wang L, Song W, Ma T, Gu J, Li W (2019) Core fucosylation of copper transporter 1 plays a crucial role in cisplatin-resistance of epithelial ovarian cancer by regulating drug uptake. Mol Carcinog 58:1–14. https://doi.org/10.1002/mc.22971
Anugraham M, Jacob F, Nixdorf S, Everest-Dass AV, Heinzelmann-Schwarz V, Packer NH (2014) Specific glycosylation of membrane proteins in epithelial ovarian cancer cell lines: glycan structures reflect gene expression and DNA methylation status. Mol Cell Proteomics 13(9):2213–2232. https://doi.org/10.1074/mcp.M113.037085
Medzihradszky KF, Kaasik K, Chalkley RJ (2010) Tissue-specific glycosylation at the Glycopeptide level. Mol Cell Proteomics 14(8):2103–2110. https://doi.org/10.1074/mcp.M115.050393
Aoyagi Y, Isemura M, Yosizawa Z, Suzuki Y, Sekine C, Ono T, Ichida F (1985) Fucosylation of serum alpha fetoprotein in patients with primary hepatocellular carcinoma. Biochim Biophys Acta 830(3):217–223
Noda K, Miyoshi E, Kitada T, Nakahara S, Gao CX, Honke K, Shiratori Y, Moriwaki H, Sasaki Y, Kasahara A, Hori M, Hayashi N, Taniguchi N (2002) The enzymatic basis for the conversion of non-fucosylated to fucosylated alpha-fetoprotein by acyclic retinoid treatment in human hepatoma cells: activation of alpha 1,6 fucosyltransferase. Tumor Biol 23(4):202–211
Yan LM, Lin B, Zhu LC, Hao YY, Qi Y, Wang CZ, Gao S, Liu SC, Zhang SL, Iwamori M (2010) Enhancement of the adhesive and spreading potentials of ovarian carcinoma rmg-1 cells due to increased expression of integrin alpha5beta1 with the Lewis y-structure on transfection of the alpha1,2-fucosyltransferase gene. Biochim 92(7):852–857. https://doi.org/10.1016/j.biochi.2010.02.012
Gao L, Yan L, Lin B, Gao J, Liang X, Wang Y, Liu J, Zhang S, Iwamori M (2011) Enhancive effects of Lewis y antigen on cd44-mediated adhesion and spreading of human ovarian cancer cell line rmg-i. J Exp Clin Cancer Res 30:15. https://doi.org/10.1186/1756-9966-30-15
Wang X, Gu J, Ihara H, Miyoshi E, Honke K, Taniguchi N (2006) Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. J Biol Chem 281(5):2572–2577
Orczyk-Pawilowicz M (2007) The role of fucosylation of glycoconjugates in health and disease. Postepy Hig Med Dosw (Online) 61:240–252
Mehta A, Comunale MA, Rawat S, Casciano JC, Lamontagne J, Herrera H, Ramanathan A, Betesh L, Wang M, Norton P, Steel LF, Bouchard MJ (2016) Intrinsic hepatocyte dedifferentiation is accompanied by upregulation of mesenchymal markers, protein sialylation and core alpha 1,6 linked fucosylation. Sci Rep 6:27965. https://doi.org/10.1038/srep27965
Wang H, Deng L, Cai M, Zhuang H, Zhu L, Hao Y, Gao J, Liu J, Li X, Lin B (2016) Annexin A4 fucosylation enhances its interaction with the NF-kB p50 and promotes tumor progression of ovarian clear cell carcinoma. Oncotarget 8(64):108093–108107. https://doi.org/10.18632/oncotarget.10226
Mitra I, Alley WR Jr, Goetz JA, Vasseur JA, Novotny MV, Jacobson SC (2013) Comparative profiling of N-glycans isolated from serum samples of ovarian cancer patients and analyzed by microchip electrophoresis. Proteome Res 12(10):4490–4496. https://doi.org/10.1021/pr400549e
Wang Y, Fukuda T, Isaji T, Lu J, Im S, Hang Q, Gu W, Hou S, Ohtsubo K, Gu J (2015) Loss of α1,6-fucosyltransferase inhibits chemical-induced hepatocellular carcinoma and tumorigenesis by down-regulating several cell signaling pathways. FASEB J 29(8):3217–3227. https://doi.org/10.1096/fj.15-270710
Phillips T (2008) Regulation of transcription and gene expression in eukaryotes. Nature Education 1(1):199
Prendergast JM, Galvao da Silva AP, Eavarone DA, Ghaderi D, Zhang M, Brady D, Wicks J, DeSander J, Behrens J, Rueda BR (2017) Novel anti-Sialyl-Tn monoclonal antibodies and antibody-drug conjugates demonstrate tumor specificity and anti-tumor activity. MAbs 9(4):615–627. https://doi.org/10.1080/19420862.2017.1290752
Bassoy EY, Kasahara A, Chiusolo V, Jacquemin G, Boydell E, Zamorano S, Riccadonna C, Pellegatta S, Hulo N, Dutoit V, Derouazi M, Dietrich PY, Walker PR, Martinvalet D (2017) ER-mitochondria contacts control surface glycan expression and sensitivity to killer lymphocytes in glioma stem-like cells. EMBO 36(11):1493–1512. https://doi.org/10.15252/embj.201695429
Sato Y, Isaji T, Tajiri M, Yoshida-Yamamoto S, Yoshinaka T, Somehara T, Fukuda T, Wada Y, Gu J (2009) An N-glycosylation site on the β-propeller domain of the integrin α5 subunit plays key roles in both its function and site-specific modification by β1, 4-N-acetylglucosaminyltransferase III. J Biol Chem 284(18):11873–11881. https://doi.org/10.1074/jbc.M807660200
Shields RL, Lai J, Keck R, O'Connell LY, Hong K, Meng YG, Weikert SH, Presta LG (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human fc gamma RIII and antibody-dependent cellular toxicity. J Biol Chem 277(30):26733–26740
Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K (2003) The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 278(5):3466–3473
Acknowledgments
We gratefully acknowledge Gabriel Martinez Batallar for mass spectrometry analysis at the laboratory of Dr. Sergio Encarnación Guevara from Centro de Ciencias Genómicas, UNAM, Mexico; Jessica Márquez Dueñas for her help in the purchase and procurement of materials.
Funding
This project was supported by a grant for Health Research (233739), from CONACYT and the Health Ministry, México; DRAA was a recipient of a PhD fellowship also from CONACYT, México (338858).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure 1
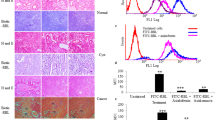
Time course of fucosylation of SKOV-3 cells treated with ascites 01 and 02. The graphs show the time course of fucosylation (arbitrary units of fluorescence expressed as fluorescence intensity) in nonpermeabilized (left panel) and permeabilized (right panel) cells. The data of three independent experiments were analyzed by one-way ANOVA in GraphPad Prism 7. (JPG 45 kb)
Supplementary Figure 2
Ascites modulate fucosylation expression in different cell lines. Membrane fucosylation was analyzed in SKOV-3 (a), OVCAR-3 (b), and Caco-2 and BEAS-2B (d) cell lines. Ascites from 10 different ovarian cancer patients were employed to stimulate the cells for 24 h. The samples were analyzed by confocal microscopy. Fucosylation was detected by biotinylated Aleuria aurantia lectin (1:200 dilution) and FITC-conjugated streptavidin (1:200 dilution). The nuclei were stained with DAPI. The selected images are representative of two independent biological replicates. The graphs show the level of fucosylation (arbitrary units of fluorescence expressed as fluorescence intensity) in SKOV-3 cells (a, lower panel) and OVCAR-3 cells (b, lower panel). The data of two independent experiments were analyzed by one-way ANOVA in GraphPad Prism 7. (JPG 2277 kb)
Supplementary Figure 3
Schematic representation of probable sites of N-glycosylation. By in silico analysis, the probable N-glycosylation sites were predicted using the NetNGlyc database. Each of the analyzed proteins is represented (each canonical isoform); the red arrows indicate the site in which the probable glycosylation may occur. The position and the value assigned are indicated next to the arrows. A potential crossing 0.5 threshold predicts glycosylation. (JPG 112 kb)
Supplementary Table 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Alberto-Aguilar, D.R., Hernández-Ramírez, V.I., Osorio-Trujillo, J.C. et al. Ascites from Ovarian Cancer Induces Novel Fucosylated Proteins. Cancer Microenvironment 12, 181–195 (2019). https://doi.org/10.1007/s12307-019-00227-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-019-00227-z