Abstract
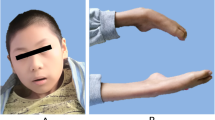
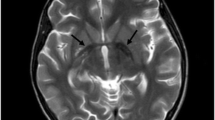
Celia’s encephalopathy (progressive encephalopathy with/without lipodystrophy (PELD)) is a childhood neurodegenerative disorder with a fatal prognosis before the age of 10, due to the variant c.985C>T in the BSCL2 gene that causes a cryptic splicing site leading to skipping of exon 7. For years, different authors have reported cases of congenital generalized lipodystrophy due to the variant c.974dupG in BSCL2 associated with neurological manifestations of variable severity, although some of them clearly superimposable to PELD. To identify the molecular mechanisms responsible for these neurological alterations in two patients with c.974dupG. Clinical characterization, biochemistry, and neuroimaging studies of two girls carrying this variant. In silico analysis, PCR amplification, and BSCL2 cDNA sequencing. BSCL2-201 transcript expression, which lacks exon 7, by qPCR in fibroblasts from the index case, from a healthy child as a control and from two patients with PELD, and in leukocytes from the index case and her parents. One with a severe encephalopathy including a picture of intellectual deficiency, severe language impairment, myoclonic epilepsy, and lipodystrophy as described in PELD, dying at 9 years and 9 months of age. The other 2-year-old patient showed incipient signs of neurological involvement. In silico and cDNA sequencing studies showed that variant c.974dupG gives rise to skipping of exon 7. The expression of BSCL2-201 in fibroblasts was significantly higher in the index case than in the healthy child, although less than in the case with homozygous PELD due to c.985C>T variant. The expression of this transcript was approximately half in the healthy carrier parents of this patient. The c.974dupG variant leads to the skipping of exon 7 of the BSCL2 gene and is responsible for a variant of Celia’s encephalopathy, with variable phenotypic expression.



Similar content being viewed by others
References
Wang H, Becuwe M, Housden BE, Chitraju C, Porras AJ, Graham MM, Liu XN, Thiam AR, Savage DB, Agarwal AK, Garg A, Olarte MJ, Lin Q, Fröhlich F, Hannibal-Bach HK, Upadhyayula S, Perrimon N, Kirchhausen T, Ejsing CS, Walther TC, Farese RV Jr (2016) Seipin is required for converting nascent to mature lipid droplets. Elife 5:e16582
Cartwright BR, Goodman JM (2012) Seipin: from human disease to molecular mechanism. J Lipid Res 53(6):1042–1055
Sanchez-Iglesias S et al (2019) Does seipin play a role in oxidative stress protection and peroxisome biogenesis? New insights from human brain autopsies. Neuroscience 396:119–137
Lundin C, Nordström R, Wagner K, Windpassinger C, Andersson H, von Heijne G, Nilsson IM (2006) Membrane topology of the human seipin protein. FEBS Lett 580(9):2281–2284
Guillen-Navarro E et al (2013) A new seipin-associated neurodegenerative syndrome. J Med Genet 50(6):401–409
Ruiz-Riquelme A, Sánchez-Iglesias S, Rábano A, Guillén-Navarro E, Domingo-Jiménez R, Ramos A, Rosa I, Senra A, Nilsson P, García Á, Araújo-Vilar D, Requena JR (2015) Larger aggregates of mutant seipin in Celia’s encephalopathy, a new protein misfolding neurodegenerative disease. Neurobiol Dis 83:44–53
Brown RJ, Araujo-Vilar D, Cheung PT, Dunger D, Garg A, Jack M, Mungai L, Oral EA, Patni N, Rother KI, von Schnurbein J, Sorkina E, Stanley T, Vigouroux C, Wabitsch M, Williams R, Yorifuji T (2016) The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab 101(12):4500–4511
Araujo-Vilar D, Santini F (2018) Diagnosis and treatment of lipodystrophy: a step-by-step approach. J Endocrinol Investig
Alaei MR et al (2016) Whole exome sequencing reveals a BSCL2 mutation causing progressive encephalopathy with lipodystrophy (PELD) in an Iranian pediatric patient. Iran Biomed J 20(5):295–301
Magre J et al (2001) Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet 28(4):365–370
Auer-Grumbach M, Schlotter-Weigel B, Lochmüller H, Strobl-Wildemann G, Auer-Grumbach P, Fischer R, Offenbacher H, Zwick EB, Robl T, Hartl G, Hartung HP, Wagner K, Windpassinger C, the Austrian Peripheral Neuropathy Study Group (2005) Phenotypes of the N88S Berardinelli-Seip congenital lipodystrophy 2 mutation. Ann Neurol 57(3):415–424
Irobi J et al (2004) The phenotype of motor neuropathies associated with BSCL2 mutations is broader than Silver syndrome and distal HMN type V. Brain 127(Pt 9):2124–2130
Agarwal AK, Arioglu E, de Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A (2002) AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet 31(1):21–23
Kim CA, Delépine M, Boutet E, el Mourabit H, le Lay S, Meier M, Nemani M, Bridel E, Leite CC, Bertola DR, Semple RK, O’Rahilly S, Dugail I, Capeau J, Lathrop M, Magré J (2008) Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab 93(4):1129–1134
Hayashi YK, Matsuda C, Ogawa M, Goto K, Tominaga K, Mitsuhashi S, Park YE, Nonaka I, Hino-Fukuyo N, Haginoya K, Sugano H, Nishino I (2009) Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest 119(9):2623–2633
Van Maldergem L et al (2002) Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J Med Genet 39(10):722–733
Agarwal AK, Simha V, Oral EA, Moran SA, Gorden P, O’Rahilly S, Zaidi Z, Gurakan F, Arslanian SA, Klar A, Ricker A, White NH, Bindl L, Herbst K, Kennel K, Patel SB, al-Gazali L, Garg A (2003) Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab 88(10):4840–4847
Wu YR, Hung SI, Chang YC, Chen ST, Lin YL, Chung WH (2009) Complementary mutations in seipin gene in a patient with Berardinelli-Seip congenital lipodystrophy and dystonia: phenotype variability suggests multiple roles of seipin gene. J Neurol Neurosurg Psychiatry 80(10):1180–1181
Huang HH, Chen TH, Hsiao HP, Huang CT, Wang CC, Shiau YH, Chao MC (2010) A Taiwanese boy with congenital generalized lipodystrophy caused by homozygous Ile262fs mutation in the BSCL2 gene. Kaohsiung J Med Sci 26(11):615–620
Opri R, Fabrizi GM, Cantalupo G, Ferrarini M, Simonati A, Dalla Bernardina B, Darra F (2016) Progressive myoclonus epilepsy in congenital generalized lipodystrophy type 2: report of 3 cases and literature review. Seizure 42:1–6
Sambrook J and Russell DW, Molecular cloning: a laboratory manual. 3rd ed. 2001, Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press
Victoria B, Cabezas-Agrícola JM, González-Méndez B, Lattanzi G, del Coco R, Loidi L, Barreiro F, Calvo C, Lado-Abeal J, Araújo-Vilar D (2010) Reduced adipogenic gene expression in fibroblasts from a patient with type 2 congenital generalized lipodystrophy. Diabet Med 27(10):1178–1187
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25(4):402–408
Araujo-Vilar D et al (2018) Association of metreleptin treatment and dietary intervention with neurological outcomes in Celia's encephalopathy. Eur J Hum Genet 26(3):396–406
Poisson A et al (2018) Regressive autism spectrum disorder expands the phenotype of BSCL2/seipin-associated neurodegeneration. Biol Psychiatry S0006–3223(18):31524–31525
Choi BO, Park MH, Chung KW, Woo HM, Koo H, Chung HK, Choi KG, Park KD, Lee HJ, Hyun YS, Koo SK (2013) Clinical and histopathological study of Charcot-Marie-tooth neuropathy with a novel S90W mutation in BSCL2. Neurogenetics 14(1):35–42
Serino D, Davico C, Specchio N, Marras CE, Fioretto F (2019) Berardinelli-Seip syndrome and progressive myoclonus epilepsy. Epileptic Disord 21(1):117–121
Acknowledgements
We are indebted to the patients and their parents for their collaboration in this study. Control 18F-FDG PET brain of subject at 7 years of age was a courtesy of A. Niñerola-Baizán and X. Setoain from Hospital Clínic Barcelona.
Funding
This work was supported by the Instituto de Salud Carlos III and the European Regional Development Fund, FEDER (grant numbers PI10/02873 and PI13/00314), by the Consellería de Industria, Xunta de Galicia (grant numbers 10PXIB208013PR and ED341b 2017/19), and by Fundación Mutua Madrileña (Call 2015). S.S-I was awarded a Research Fellowship, granted by the Asociación Española de Familiares y Afectados de Lipodistrofias (AELIP). Evaluation of case 2 was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The regional IRBs approved this study, which was conducted according to the ethical guidelines of the Helsinki Declaration.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 2480 kb)
Rights and permissions
About this article
Cite this article
Sánchez-Iglesias, S., Crocker, M., O’Callaghan, M. et al. Celia’s encephalopathy and c.974dupG in BSCL2 gene: a hidden change in a known variant. Neurogenetics 20, 73–82 (2019). https://doi.org/10.1007/s10048-019-00574-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-019-00574-5