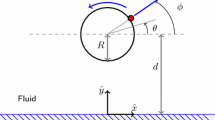
Abstract
We numerically study the effect of solid boundaries on the swimming behavior of a motile microorganism in viscoelastic media. Understanding the swimmer-wall hydrodynamic interactions is crucial to elucidate the adhesion of bacterial cells to nearby substrates which is precursor to the formation of the microbial biofilms. The microorganism is simulated using a squirmer model that captures the major swimming mechanisms of potential, extensile, and contractile types of swimmers, while neglecting the biological complexities. A Giesekus constitutive equation is utilized to describe both viscoelasticity and shear-thinning behavior of the background fluid. We found that the viscoelasticity strongly affects the near-wall motion of a squirmer by generating an opposing polymeric torque which impedes the rotation of the swimmer away from the wall. In particular, the time a neutral squirmer spends at the close proximity of the wall is shown to increase with polymer relaxation time and reaches a maximum at Weissenberg number of unity. The shear-thinning effect is found to weaken the solvent stress and therefore, increases the swimmer-wall contact time. For a puller swimmer, the polymer stretching mainly occurs around its lateral sides, leading to reduced elastic resistance against its locomotion. The neutral and puller swimmers eventually escape the wall attraction effect due to a releasing force generated by the Newtonian viscous stress. In contrast, the pusher is found to be perpetually trapped near the wall as a result of the formation of a highly stretched region behind its body. It is shown that the shear-thinning property of the fluid weakens the wall-trapping effect for the pusher squirmer.


















Similar content being viewed by others
References
Alldredge AL, Passow U , Logan BE (1993) The abundance and significance of a class of large, transparent organic particles in the ocean. Deep-Sea Res Pt I 40:1131–1140
Ardekani A , Gore E (2012) Emergence of a limit cycle for swimming microorganisms in a vortical flow of a viscoelastic fluid. Phys Rev E 85:056,309
Ardekani AM, Rangel R (2008) Numerical investigation of particle-particle and particle-wall collisions in a viscous fluid. J Fluid Mech 596:437–466
Ardekani AM, Rangel RH, Joseph DD (2007) Motion of a sphere normal to a wall in a second-order fluid. J Fluid Mech 587:163–172
Ardekani AM, Dabiri S, Rangel RH (2008) Collision of multi-particle and general shape objects in a viscous fluid . J Comput Phys 227:10,094–10,107
Ardekani AM, Joseph DD, Dunn-Rankin D, Rangel RH (2009) Particle-wall collision in a viscoelastic fluid. J Fluid Mech 633:475–483
Azam F (1992) Intense hydrolytic enzyme activity on marine aggregates and implications for rapid particle dissolution. Nature 359:10
Berg HC, Turner L (1979) Movement of microorganisms in viscous environments. Nature 278:349–351
Blake JR (1971) A spherical envelope approach to ciliary propulsion. J Fluid Mech 46:199–208
Bozorgi Y , Underhill PT (2011) Effect of viscoelasticity on the collective behavior of swimming microorganisms. Phys Rev E 84:061,901
Bozorgi Y, Underhill PT (2013) Role of linear viscoelasticity and rotational diffusivity on the collective behavior of active particles. J Rheol 57:511–533
Brennen C, Winet H (1977) Fluid mechanics of propulsion by cilia and flagella. Ann Rev Fluid Mech 9:339–398
Burrows LL (2012) Pseudomonas aeruginosa twitching motility: Type IV pili in action. Ann Rev Microbiol 66:493–520
Cunliffe M, Engel A, Frka S, Gasparovic B, Guitart C, Murrell JC, Salter M, Stolle C, Upstill-Goddard R, Wurlm O (2013) Sea surface microlayers: A unified physicochemical and biological perspective of the air-ocean interface. Prog Oceanogr 109:104–116
D’Avino G, Cicale G, Hulsen MA, Greco F, Maffettone PL (2009) Effects of confinement on the motion of a single sphere in a sheared viscoelastic liquid. Journal of Non-Newtonian Fluid Mechanics 157:101–107
Despeyroux A, Ambari A (2012) Slow motion of a sphere towards a plane through confined non-newtonian fluid. J. Non-Newtonian Fluid Mech 167:38–45
DiLuzio WR , Turner L, Mayerm M , Garstecki P, Weibel DB, Berg HC, Whitesides GM (2005) Escherichia coli swim on the right-hand side. Nature 435:1271–1274
Doostmohammadi A, Stocker R, Ardekani AM (2012) Low-Reynolds-number swimming at pycnoclines. Proc Natl Acad Sci USA 109:3856–3861
Drescher K, Dunkel J, Cisneros LH, Ganguly S, Goldstein RE (2011) Fluid dynamics and noise in bacterial cell–cell and cell–surface scattering. Proc National Academy Sci 108(27):10,940–10,945
Eisenberg DA, Klink IM, Phillips RJ (2013) Axisymmetric sedimentation of spherical particles in a viscoelastic fluid: Sphere-wall and sphere-sphere interactions. J Rheol 57:857–880
Giesekus H (1982) A simple constitutive equation for polymer fluids based on the concept of deformation-dependent tensorial mobility. J Non-Newtonian Fluid Mech 11:69–109
Glowinski R, Pan TW, Hesla TI, Joseph DD, Periaux J (2001) A fictitious domain approach to the direct numerical simulation of incompressible viscous flow past moving rigid bodies: application to particulate flow. J Comput Phys 1690:363–426
Goyal N , Derksen JJ (2012) Direct simulations of spherical particles sedimenting in viscoelastic fluids. J Non-Newton Fluid Mech 183:1–13
Guenette R, Fortin M (1995) A new mixed finite element method for computing viscoelastic flows. J Non-Newtonian Fluid Mech 60:27–52
Hall-Stoodley L, Costerton JW, Stoodley P (2004 ) Bacterial biofilms: from the Natural environment to infectious diseases. Nature Rev Microbiol 2:95–108
Harman MW, Dunham-Ems SM, Caimano MJ, Belperron AA, Bockenstedt LK, Fu HC, Radolf JD, Wolgemuth CW (2012) The heterogeneous motility of the Lyme disease spirochete in gelatin mimics dissemination through tissue. Proc Natl Acad Sci USA 109:3059
Houry A, Briandet R, Aymerich S, Gohar M (2010) Involvement of motility and flagella in Bacillus cereus biofilm formation. Microbiol 156:1009–1018
Ishikawa T, Simmonds MP, Pedley TJ (2006) Hydrodynamic interaction of two swimming model micro-organisms. J Fluid Mech 568:119–160
Ishimoto K, Gaffney EA (2013) Squirmer dynamics near a boundary. Phys Rev E 88:062–702
Jung S (2010) Caenorhabditis elegans swimming in a saturated particulate system. Phys Fluids 22:031–903
Kim TJ, Young BM , Young GM (2008 ) Effect of flagellar mutations on Yersinia enterocolitica biofilm formation. Appl Environ Microbiol 74:5466–5474
Kimsey RB, Spielman A (1990) Motility of Lyme Disease Spirochetes in Fluids as Viscous as the Extracellular Matrix. J Infect Dis 162:1205–1208
Klapper I, Rupp CJ , Cargo R , Purvedorj B , Stoodley P (2002) Viscoelastic fluid description of bacterial biofilm material properties. Biotechnol Bioeng 80:289–296
Lauga E (2007) Propulsion in a viscoelastic fluid. Phys Fluids 19:083,104
Lauga E (2009) Life at high deborah number. Europhysics Letters 86(6):64,001
Lemon KP, Higgins DE, Kolter R (2007) Flagellar Motility Is Critical for Listeria monocytogenes Biofilm Formation. J Bacteriol 189:4418–4424
Leonard BP (1979) A stable and accurate convective modelling procedure based on quadratic upstream interpolation. Comput Meth Appl Mech Engng 19:59–98
Li G, Ardekani AM (2014) Hydrodynamic interaction of microswimmers near a wall. Phys Rev E 90:013,010
Li G, Tam LK, Tang JX (2008) Amplified effect of brownian motion in bacterial near-surface swimming. Proc National Academy Sci USA 105:18,359–35,518
Lighthill MJ (1952) On the squirming motion of nearly spherical deformable bodies through liquids at very small Reynolds numbers. Comm Pure Appl Math 5:109–118
Liu B, Powers TR, Breuer KS (2011) Force-free swimming of a model helical flagellum in viscoelastic fluids. Proc Natl Acad Sci USA 108:19,516–19,520
Llopis I, Pagonabarraga I (2010) Hydrodynamic interactions in squirmer motion: Swimming with a neighbour and close to a wall. J Non-Newtonian Fluid Mec 165:946–952
Merritt PM, Danhorn T, Fuqua C (2007) Motility and Chemotaxis in Agrobacterium tumefaciens Surface Attachment and Biofilm Formation. J Bacteriol 189:8005–8014
Montecucco C, Rappuoli R (2001) Living dangerously: How Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol 2:457–466
O’Toole GA, Kolter R (1998) Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol 30:295–304
Padhy S, Rodriguez M, Shaqfeh ESG, Iaccarino G, Morris JF, Tonmukayakul N (2013) The effect of shear thinning and walls on the sedimentation of a sphere in an elastic fluid under orthogonal shear. J Non-Newtonian Fluid Mech 201:120–129
Passow U (2002) Transparent exopolymer particles (TEP) in aquatic environments. Prog Oceanogr 55:287–333
Pratt LA, Kolter R (1998) Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol 30:285–293
Schleiniger G, Weinacht RJ (1991) A remark on the Giesekus viscoelastic fluid. J Rheol 35:1157–1170
Shaw T, Winston M, Rupp CJ, Klapper I, Stoodley P (2004) Commonality of Elastic Relaxation Times in Biofilms. Phys Rev Lett 93:098,102
Shen XN, Arratia PE (2011) Undulatory Swimming in Viscoelastic Fluids. Phys Rev Lett 106:208,101
Snijkers F, D’Avino G, Maffettone PL, Greco F (2011) Effect of viscoelasticity on the rotation of a sphere in shear flow. J Non-Newton Fluid Mech 166:363–372
Spagnolie SE, Lauga E (2012) Hydrodynamics of self-propulsion near a boundary: predictions and accuracy of far-field approximations. J Fluid Mech 700:105–147
Spagnolie SE, Liu B, Powers TR (2013) Locomotion of Helical Bodies in Viscoelastic Fluids: Enhanced Swimming at Large Helical Amplitudes. Phys Rev Lett 111:068,101
Suarez SS, Pacey AA (2006) Sperm transport in the female reproductive tract. Human Reprod Update 12:23–37
Tatum JA, Finnis MV, Lawson NJ, Harrison GM (2007) 3D particle image velocimetry of the flow field around a sphere sedimenting near a wall: Part 1. Effects of Weissenberg number. J non-Newtonian Fluid Mech 141(2):99– 115
Taylor G (1951) Analysis of the swimming of microscopic organisms. Proceedings of the Royal Society of London Series A-Mathematical. Phys Sci 209:447–461
Teran J, Fauci L, Shelley M (2010) Viscoelastic Fluid Response Can Increase the Speed and Efficiency of a Free Swimmer. Phys Rev Lett 104:038,101
Thutupalli S, Seemann R, Herminghaus S (2011) Swarming behavior of simple model squirmers. New J. Phys 13(7):073–021
Tolker-Nielsen T, Brinch UC, Ragas PC, Andersen JB, Jacobsen CS, Molin S (2000) Development and dynamics of Pseudomonas sp. biofilms. J Bacteriol 182:6482–6489
Tuson HH, Weibel DB (2013) Bacteria-surface interactions. Soft Matter 9:4368–4380
Vlamakis H, Aguilar C, Losick R, Kolter R (2008) Control of cell fate by the formation of an architecturally complex bacterial community. Genes Dev 22:945–953
Wang S, Ardekani A (2013) Ciliates swimming near an interface. Phys Rev E 87:063010
Wang S, Ardekani AM (2012a) Inertial squirmer. Phys Fluids 24:101,902
Wang S, Ardekani AM (2012b) Unsteady swimming of small organisms. J Fluid Mech 702:286–297
Watnick PI, Kolter R (1999) Steps in the development of a Vibrio cholerae El Tor biofilm. Mol Microbiol 34:586–595
Wloka M, Rehage H, Flemming HC, Wingender J (2012) Rheological properties of viscoelastic biofilm extracellular polymeric substances and comparison to the behavior of calcium alginate gels. Colloid Polym Sci 282:1067–1076
Wolgemuth CW, Charon NW, Goldstein SF, Goldstein RE (2006) The flagellar cytoskeleton of the spirochetes. J Mol Microbiol Biotechnol 11:221–227
Zhu L, Do-Quang M, Lauga E, Brandt L (2011) Locomotion by tangential deformation in a polymeric fluid. Phys Rev E 83:011,901
Zhu L, Lauga E, Brandt L (2012) Self-propulsion in viscoelastic fluids: Pushers vs. pullers. Phys Fluids 24:051,902
Acknowledgements
This publication was made possible, in part, with support from NSF (Grant No. CBET- 1150348-CAREER) and Indiana Clinical and Translational Sciences Institute Collaboration in Biomedical/Translational Research (CBR/CTR) Pilot Program Grants (Grant No. TR000006) from the National Institute of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue devoted to novel trends in rheology.
Appendix: Verification of the numerical method
Appendix: Verification of the numerical method
A.1 Rotation of a single sphere in an Oldroyd-B shear flow
In an Oldroyd-B fluid, we simulate the rotation of a single sphere in a shear flow to verify our numerical platform. Simulation is conducted in a rectangular domain of [−2a,2a] × [−2a, 2a] × [−4a, 4a] where a is the radius of the sphere and the sphere is centered at (0,0,0). The flow is driven by two parallel plates at z = −4a and z = 4a moving opposite in x-direction with the same speed U. Periodic boundary conditions are applied in x and y directions. The mesh size is Δ=a/16 and the time step is Δt = 10−3 a/U. The shear rate of the flow is \(\dot {\gamma }=U/4a\), the Weissenberg number \(Wi=\lambda \dot {\gamma }\), the Reynolds number \(Re=\rho \dot {\gamma } a^{2}/\mu =0.025\) and the viscosity ratio β s = 0.5. Figure 19a shows the time evolution of the angular velocity of the sphere at different W i. It is seen that for the Newtonian case, the sphere asymptotically reaches to its steady state of \({\Omega }_{y}=0.5\dot {\gamma }\) while for viscoelastic cases, overshoots can be observed around \(t\dot {\gamma }=0.2\). In Fig. 19b, the steady angular velocity as a function of W i is compared with previous experimental (Snijkers et al. 2011) and numerical (Goyal and Derksen 2012) results. It is evident that our simulation results are in good agreement with the previous results.
A.2 Free swimming of a squirmer in a Giesekus fluid in an unbounded domain
The simulation is performed on a non-uniform structured grid with the smallest mesh size of Δ = D/40 near the squirmer, where D is the diameter of the spherical squirmer. The computational domain is [−40a, 40a] × [−40a, 40a] × [−40a, 40a] and the squirmer is initially placed at (0,0,0). The time step is Δt = 10−5. The Reynolds number, defined as R e = U 0 a/ν, is 0.01 in all the simulations, and U 0 = 2B 1/3. According to the analysis of a squirmer in a Newtonian fluid at finite Reynolds number, the swimming speed of a squirmer is determined by U/U 0 ≃ 1 − 0.15β R e (Wang and Ardekani 2012a), thus the effects of the inertia on the swimming speed can be neglected in our simulation. The viscosity ratio is β s = 0.5 and mobility factor is α m = 0.2. The Weissenberg number is defined as W i = λ B 1/a. The swimming speed of the squirmer U is plotted in Fig. 20 for squirmers with β = −5, 0, and 5. Our results show good agreement with the results obtained by Zhu et al. (2012).
(Color online) Swimming speed U as a function of the Weissenberg number W i, for the neutral squirmer β = 0 (solid line: (Zhu et al. 2012) and circles: present results), pusher β = −5 (dashed line: (Zhu et al. 2012) and squares: present results) and puller β = 5 (dashdot line: (Zhu et al. 2012) and triangles: present results). The Reynolds number is R e = 0.01 and the swimming speed is scaled by the squirmer’s speed U 0 in a Newtonian fluid
A.3 Convergence study
Convergence studies have been performed for the near-wall motion of squirmers with β = 0 and −3 under different grid sizes and different time steps. Figure 21 shows the time history of the distance h away from the wall, orientation angle α and the swimming speed U of the squirmer. The results from these different computations agree well with each other. It is confirmed that the computed results are independent of the mesh size and the time step.
(Color online) Time history of a vertical distance h and orientation angle α and b swimming speed U of the neutral squirmer calculated using different grid sizes, different time steps and different values of the parameter 𝜖. The corresponding parameters are W i = 6 and R e = 0.1 and the squirmer is initialized at h 0 = 2 and α 0 = −π/4
A.4 Repulsive force
When the squirmer lies in the close proximity of the surface, due to the lubrication effect and other non-hydrodynamic phenomena such as electrostatic charges, a repulsive force is developed which prevents intrusion of the swimmer’s body into the wall. To capture the associated hydrodynamic squeezing effect, exceedingly fine grid resolutions are needed which make the corresponding simulations computationally highly demanding. In addition, as indicated by Spagnolie and Lauga (2012), hydrodynamic interactions are inadequate to prevent the swimmer-wall interference in some settings. Hence, in order to avoid overlapping of the squirmer’s body and the nearby wall, we impose a short-range repulsive force (Glowinski et al. 2001) defined as,
where h min = a is the minimum possible distance from the wall and h r represents the range over which the force is acting and is normally set to be the smallest grid size Δ in the computational domain (Glowinski et al. 2001). The direction of the repulsive force e is considered to be perpendicular to the wall. The parameters \(C_{m}=M_{p} {U_{0}^{2}} /a\) and 𝜖 = 10−4 denote a scaling factor and a small positive number, respectively, with M p being the mass of the squirmer. As demonstrated in Fig. 21, changing the value of 𝜖 have a negligible impact on the simulation results.
Rights and permissions
About this article
Cite this article
Li, G.J., Karimi, A. & Ardekani, A.M. Effect of solid boundaries on swimming dynamics of microorganisms in a viscoelastic fluid. Rheol Acta 53, 911–926 (2014). https://doi.org/10.1007/s00397-014-0796-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00397-014-0796-9