Abstract
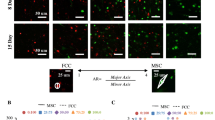
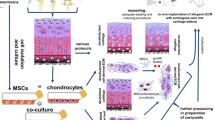
Decellularized matrix (DCM) derived from native tissues may be a promising supporting material to induce cellular differentiation by sequestered bioactive factors. However, no previous study has investigated the use of human meniscus-derived DCM to re-differentiate human meniscus fibrochondrocytes (MFCs) to form meniscus-like extracellular matrix (ECM). We expanded human MFCs and seeded them upon a cadaveric meniscus-derived DCM prepared by physical homogenization under hypoxia. To assess the bioactivity of the DCM, we used conditions with and without chondrogenic factor TGF-β3 and set up a cell pellet culture model as a biomaterial-free control. We found that the DCM supported chondrogenic re-differentiation and ECM formation of MFCs only in the presence of exogenous TGF-β3. Chondrogenic re-differentiation was more robust at the protein level in the pellet model as MFCs on the DCM appeared to favour a more proliferative phenotype. Interestingly, without growth factors, the DCM tended to promote expression of hypertrophic differentiation markers relative to the pellet model. Therefore, the human meniscus-derived DCM prepared by physical homogenization contained insufficient bioactive factors to induce appreciable ECM formation by human MFCs.





Similar content being viewed by others
References
Abdelgaied, A., M. Stanley, M. Galfe, H. Berry, E. Ingham, and J. Fisher. Comparison of the biomechanical tensile and compressive properties of decellularised and natural porcine meniscus. J. Biomech. 48:1389–1396, 2015.
Adesida, A. B., L. M. Grady, W. S. Khan, and T. E. Hardingham. The matrix-forming phenotype of cultured human meniscus cells is enhanced after culture with fibroblast growth factor 2 and is further stimulated by hypoxia. Arthritis Res. Ther. 8:R61, 2006.
Adesida, A. B., A. Mulet-Sierra, and N. M. Jomha. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res. Ther. 3:9, 2012.
Azhim, A., T. Ono, Y. Fukui, Y. Morimoto, K. Furukawa, and T. Ushida. Preparation of decellularized meniscal scaffolds using sonication treatment for tissue engineering. In: 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE, 2013, pp. 6953–6956.
Baker, B. M., and R. L. Mauck. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials 28:1967–1977, 2007.
Baker, B. M., A. S. Nathan, G. R. Huffman, and R. L. Mauck. Tissue engineering with meniscus cells derived from surgical debris. Osteoarthr. Cartil. Osteoarthr. Res. Soc. 17:336–345, 2009.
Bornes, T. D., N. M. Jomha, A. Mulet-Sierra, and A. B. Adesida. Optimal seeding densities for in vitro chondrogenesis of two- and three-dimensional-isolated and-expanded bone marrow-derived mesenchymal stromal stem cells within a porous collagen scaffold. Tissue Eng. C 22:208–220, 2016.
Chen, Y. C., R. N. Chen, H. J. Jhan, D. Z. Liu, H. O. Ho, Y. Mao, et al. Development and characterization of acellular extracellular matrix scaffolds from porcine menisci for use in cartilage tissue engineering. Tissue Eng. C 21:971–986, 2015.
Chen, Y. C., R. N. Chen, H. J. Jhan, D. Z. Liu, H. O. Ho, Y. Mao, et al. Development and characterization of acellular extracellular matrix scaffolds from porcine menisci for use in cartilage tissue engineering. Tissue Eng. C 2015. https://doi.org/10.1089/ten.TEC.2015.0036.
Cheng, N. C., B. T. Estes, H. A. Awad, and F. Guilak. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng. A 15:231–241, 2009.
Cheung, H. S. Distribution of type I, II, III and V in the pepsin solubilized collagens in bovine menisci. Connect. Tissue Res. 16:343–356, 1987.
Crapo, P. M., T. W. Gilbert, and S. F. Badylak. An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243, 2011.
Gao, S., W. Guo, M. Chen, Z. Yuan, M. Wang, Y. Zhang, et al. Fabrication and characterization of electrospun nanofibers composed of decellularized meniscus extracellular matrix and polycaprolactone for meniscus tissue engineering. J. Mater. Chem. B 5:2273–2285, 2017.
Hall, B. K., and T. Miyake. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. Int. J. Dev. Biol. 39:881–893, 2004.
Hoshiba, T., G. Chen, C. Endo, H. Maruyama, M. Wakui, E. Nemoto, et al. Decellularized extracellular matrix as an in vitro model to study the comprehensive roles of the ECM in stem cell differentiation. Stem Cells Int. 2015. https://doi.org/10.1155/2016/6397820.
Liang, Y., E. Idrees, S. H. J. Andrews, K. Labib, A. Szojka, M. Kunze, et al. Plasticity of human meniscus fibrochondrocytes: a study on effects of mitotic divisions and oxygen tension. Sci. Rep. 7:12148, 2017.
Liang, Y., E. Idrees, A. R. A. Szojka, S. H. J. Andrews, M. Kunze, A. Mulet-Sierra, et al. Chondrogenic differentiation of synovial fluid mesenchymal stem cells on human meniscus-derived decellularized matrix requires exogenous growth factors. Acta biomater. 80:131–143, 2018.
Lim, Y.-B., S.-S. Kang, T. K. Park, Y.-S. Lee, J.-S. Chun, and J. K. Sonn. Disruption of actin cytoskeleton induces chondrogenesis of mesenchymal cells by activating protein kinase C-α signaling. Biochem. Biophys. Res. Commun. 273:609–613, 2000.
Lund-Olesen, K. Oxygen tension in synovial fluids. Arthritis Rheumatol. 13:769–776, 1970.
Maier, D., K. Braeun, E. Steinhauser, P. Ueblacker, M. Oberst, P. C. Kreuz, et al. In vitro analysis of an allogenic scaffold for tissue-engineered meniscus replacement. J. Orthop. Res. 25:1598–1608, 2007.
Makris, E. A., P. Hadidi, and K. A. Athanasiou. The knee meniscus: structure–function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 32:7411–7431, 2011.
Marsano, A., C. M. Medeiros da Cunha, S. Ghanaati, S. Gueven, M. Centola, R. Tsaryk, et al. Spontaneous in vivo chondrogenesis of bone marrow-derived mesenchymal progenitor cells by blocking vascular endothelial growth factor signaling. Stem Cells Transl. Med. 5:1730–1738, 2016.
Mauck, R. L., C. C. Wang, E. S. Oswald, G. A. Ateshian, and C. T. Hung. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthr. Cartil. Osteoarthr. Res. Soc. 11:879–890, 2003.
McCorry, M. C., and L. J. Bonassar. Fiber development and matrix production in tissue-engineered menisci using bovine mesenchymal stem cells and fibrochondrocytes. Connect. Tissue Res. 58:329–341, 2017.
McDermott, I., and A. Amis. The consequences of meniscectomy. J. Bone Jt Surg. Br. 88:1549–1556, 2006.
Monibi, F. A., C. C. Bozynski, K. Kuroki, A. M. Stoker, F. M. Pfeiffer, S. L. Sherman, et al. Development of a micronized meniscus extracellular matrix scaffold for potential augmentation of meniscal repair and regeneration. Tissue Eng. C 22:1059–1070, 2016.
Monibi, F. A., and J. L. Cook. Tissue-derived extracellular matrix bioscaffolds: emerging applications in cartilage and meniscus repair. Tissue Eng. B 23:386–398, 2017.
Nakata, K., K. Shino, M. Hamada, T. Mae, T. Miyama, H. Shinjo, et al. Human meniscus cell: characterization of the primary culture and use for tissue engineering. Clin. Orthop. Relat. Res. 391:S208–S218, 2001.
Oberlender, S. A., and R. S. Tuan. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development 120:177–187, 1994.
Rothrauff, B. B., K. Shimomura, R. Gottardi, P. G. Alexander, and R. S. Tuan. Anatomical region-dependent enhancement of 3-dimensional chondrogenic differentiation of human mesenchymal stem cells by soluble meniscus extracellular matrix. Acta biomater. 49:140–151, 2017.
Sandmann, G., S. Eichhorn, S. Vogt, C. Adamczyk, S. Aryee, M. Hoberg, et al. Generation and characterization of a human acellular meniscus scaffold for tissue engineering. J. Biomed. Mater. Res. A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 91:567–574, 2009.
Scott, P. G., T. Nakano, and C. M. Dodd. Isolation and characterization of small proteoglycans from different zones of the porcine knee meniscus. Biochim. Biophys. Acta Gen. Subj. 1336:254–262, 1997.
Shimomura, K., B. B. Rothrauff, and R. S. Tuan. Region-specific effect of the decellularized meniscus extracellular matrix on mesenchymal stem cell-based meniscus tissue engineering. Am. J. Sports Med. 45:604–611, 2017.
Stapleton, T. W., J. Ingram, J. Katta, R. Knight, S. Korossis, J. Fisher, et al. Development and characterization of an acellular porcine medial meniscus for use in tissue engineering. Tissue Eng. A 14:505–518, 2008.
Szojka, A. R. A., B. D. Lyons, C. N. Moore, Y. Liang, M. Kunze, E. Idrees, et al. Hypoxia and TGF-beta3 synergistically mediate inner meniscus-like matrix formation by fibrochondrocytes. Tissue Eng. A 2018. https://doi.org/10.1089/ten.TEA.2018.0211.
Urist, M. R. Bone: formation by autoinduction. Science (NY NY) 150:893–899, 1965.
Van der Kraan, P., P. Buma, T. Van Kuppevelt, and W. Van Den Berg. Interaction of chondrocytes, extracellular matrix and growth factors: relevance for articular cartilage tissue engineering. Osteoarthr. Cartil. 10:631–637, 2002.
Wei, F., J. Zhou, X. Wei, J. Zhang, B. C. Fleming, R. Terek, et al. Activation of Indian hedgehog promotes chondrocyte hypertrophy and upregulation of MMP-13 in human osteoarthritic cartilage. Osteoarthr. Cartil. 20:755–763, 2012.
Acknowledgments
Financial support was provided by Canadian Institutes for Health Research (CIHR MOP 125921), Canadian Foundation for Innovation (CFI 33786), University Hospital Foundation (RES0028185), Natural Sciences and Engineering Research Council (NSERC RGPIN-2018-06290) to AA. Stipend support to YL was provided by Li Ka Shing Foundation. We thank the Comprehensive Tissue Centre, Alberta Health Services for acquisition and donation of human cadaver tissue specimens. We thank Dr. Jian Ling (Southwest Research Institute, San Antonio, Texas, USA) for performing ethylene oxide sterilization. We thank the Uludag Lab at the University of Alberta for assistance with freeze drying. We thank the Mr. Woo Jung Cho (Faculty of Medicine and Dentistry, Cell Imaging Center, University of Alberta, Alberta, Canada) for SEM analysis.
Author information
Authors and Affiliations
Contributions
YL conducted the experiments and was responsible for data acquisition, analysis, and bulk of manuscript writing. AS was involved in data analysis and manuscript writing. EI, MK and AMS were involved in immunofluorescence, gene expression analysis, and cell culture. ABA conceived the study, supervised the study, performed data analysis, manuscript writing, and was responsible for final review of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Associate Editor Emmanuel Opara oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, Y., Szojka, A.R.A., Idrees, E. et al. Re-Differentiation of Human Meniscus Fibrochondrocytes Differs in Three-Dimensional Cell Aggregates and Decellularized Human Meniscus Matrix Scaffolds. Ann Biomed Eng 48, 968–979 (2020). https://doi.org/10.1007/s10439-019-02272-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02272-7