Abstract
Purpose
The guidelines suggest using granulocyte-colony stimulating factor (G-CSF) for febrile neutropenia (FN) as prophylaxis in chemotherapy protocols with the risk of 10–20% after assessment of patient’s risk factors. Therefore, the aim of this study is to assess the risk of FN by using the Patient Risk Score (PRS) and evaluating G-CSF use and its side effects by a clinical pharmacist at an outpatient clinic.
Methods
The study was conducted from May 2017 until November 2017 at the University Hospital oncology outpatient clinic. Patients who receive chemotherapy protocols with FN risk of 10–20% and > 20% and were initiated G-CSF were included. The patients’ risk factors were assessed by the PRS, and the side effects were monitored for 3 months by a clinical pharmacist via a patient self-reported monitoring card.
Results
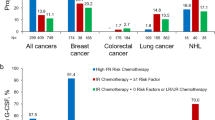
A total of 118 patients were included (286 interviews) in the study. There was a significant increase between the first and third visits on the PRS total scores of patients (p = 0.034). The patterns of G-CSF use showed that 34.7% undertreated, 22.8% overtreated, and 42.3% of patients were correctly treated for the prophylaxis. The severity of G-CSF-related musculoskeletal pain was increased on the second and third days of treatment.
Conclusions
The use of G-CSFs for FN prophylaxis is recommended; however, there may be a group of patients who are inadequately or unnecessarily treated. Therefore, patients should be assessed for the risk of developing FN in each cycle of chemotherapy and a regular risk assessment by using the PRS can be implemented in the monitoring process.



Similar content being viewed by others
References
Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, Lyman GH, Pettengell R, Tjan-Heijnen VC, Walewski J, Weber DC, Zielinski C, European Organisation for R, Treatment of C (2011) 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer 47(1):8–32. https://doi.org/10.1016/j.ejca.2010.10.013
Mitchell S, Li X, Woods M, Garcia J, Hebard-Massey K, Barron R, Samuel M (2016) Comparative effectiveness of granulocyte colony-stimulating factors to prevent febrile neutropenia and related complications in cancer patients in clinical practice: a systematic review. J Oncol Pharm Pract 22(5):702–716. https://doi.org/10.1177/1078155215625459
Silvestris N, Del Re M, Azzariti A, Maiello E, Lombardi L, Cinieri S, Guarini A, Brunetti AE, Delcuratolo S, De Vita F, Pisconti S, Danesi R, Colucci G (2012) Optimized granulocyte colony-stimulating factor prophylaxis in adult cancer patients: from biological principles to clinical guidelines. Expert Opin Ther Targets 16(Suppl 2):S111–S117. https://doi.org/10.1517/14728222.2011.652089
Crawford J, Becker, P. S., Armitage, J.O., Blayney, D. W., Cataland, S.R., Curtin, P., Fynan, T., Griffiths, E. A., Hough, S. et al. (2016) NCCN Guidelines version 2.2016 Myeloid Growth Factors
Smith TJ, Bohlke K, Armitage JO (2015) Recommendations for the use of white blood cell growth factors: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract 11(6):511–513. https://doi.org/10.1200/JOP.2015.006742
Wang L, Baser O, Kutikova L, Page JH, Barron R (2015) The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 23(11):3131–3140. https://doi.org/10.1007/s00520-015-2686-9
Lyman GH, Michels SL, Reynolds MW, Barron R, Tomic KS, Yu J (2010) Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 116(23):5555–5563. https://doi.org/10.1002/cncr.25332
Gascon P, Aapro M, Ludwig H, Bokemeyer C, Boccadoro M, Turner M, Denhaerynck K, MacDonald K, Abraham I (2016) Treatment patterns and outcomes in the prophylaxis of chemotherapy-induced (febrile) neutropenia with biosimilar filgrastim (the MONITOR-GCSF study). Support Care Cancer 24(2):911–925. https://doi.org/10.1007/s00520-015-2861-z
Lambertini M, Del Mastro L, Bellodi A, Pronzato P (2014) The five “Ws” for bone pain due to the administration of granulocyte-colony stimulating factors (G-CSFs). Crit Rev Oncol Hematol 89(1):112–128. https://doi.org/10.1016/j.critrevonc.2013.08.006
Gavioli E, Abrams M (2017) Prevention of granulocyte-colony stimulating factor (G-CSF) induced bone pain using double histamine blockade. Support Care Cancer 25(3):817–822. https://doi.org/10.1007/s00520-016-3465-y
Aapro M, Bokemeyer C, Ludwig H, Gascon P, Boccadoro M, Denhaerynck K, Gorray M, Krendyukov A, MacDonald K, Abraham I (2017) Chemotherapy-induced (febrile) neutropenia prophylaxis with biosimilar filgrastim in elderly versus non-elderly cancer patients: patterns, outcomes, and determinants (MONITOR-GCSF study). J Geriatr Oncol 8(2):86–95. https://doi.org/10.1016/j.jgo.2016.09.006
Bennett CL, Djulbegovic B, Norris LB, Armitage JO (2013) Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med 368(12):1131–1139. https://doi.org/10.1056/NEJMct1210890
Clark OA, Lyman GH, Castro AA, Clark LG, Djulbegovic B (2005) Colony-stimulating factors for chemotherapy-induced febrile neutropenia: a meta-analysis of randomized controlled trials. J Clin Oncol 23(18):4198–4214. https://doi.org/10.1200/JCO.2005.05.645
Kraj L, Krawczyk-Lipiec J, Gorniewska J, Orlik G (2017) Efficacy and safety of biosimilar filgrastim in primary and secondary prevention of febrile neutropenia. Biomed Rep 7(2):143–147. https://doi.org/10.3892/br.2017.938
Nahon S, Rastkhah M, Ben Abdelghani M, Soumoudronga RF, Gasnereau I, Labourey JL (2016) Zarzio(R), biosimilar of filgrastim, in prophylaxis of chemotherapy-induced neutropenia in routine practice: a French prospective multicentric study. Support Care Cancer 24(5):1991–1998. https://doi.org/10.1007/s00520-015-2986-0
Tesch H, Ulshofer T, Vehling-Kaiser U, Ottillinger B, Bulenda D, Turner M (2015) Prevention and treatment of chemotherapy-induced neutropenia with the biosimilar filgrastim: a non-interventional observational study of clinical practice patterns. Oncol Res Treat 38(4):146–152. https://doi.org/10.1159/000381318
Krzemieniecki K, Sevelda P, Erdkamp F, Smakal M, Schwenkglenks M, Puertas J, Trojan A, Szabo Z, Bendall K, Maenpaa J (2014) Neutropenia management and granulocyte colony-stimulating factor use in patients with solid tumours receiving myelotoxic chemotherapy--findings from clinical practice. Support Care Cancer 22(3):667–677. https://doi.org/10.1007/s00520-013-2021-2
Lyman GH, Abella E, Pettengell R (2014) Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol 90(3):190–199. https://doi.org/10.1016/j.critrevonc.2013.12.006
Barnes G, Pathak A, Schwartzberg L (2014) G-CSF utilization rate and prescribing patterns in United States: associations between physician and patient factors and GCSF use. Cancer Med 3(6):1477–1484. https://doi.org/10.1002/cam4.344
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Hacettepe University Clinical Trials Ethics Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aras, E., Bayraktar-Ekincioglu, A. & Kilickap, S. Risk assessment of febrile neutropenia and evaluation of G-CSF use in patients with cancer: a real-life study. Support Care Cancer 28, 691–699 (2020). https://doi.org/10.1007/s00520-019-04879-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-04879-x