Abstract
The surface of Hadean Earth was mainly covered with three types of rocks—komatiite, KREEP basalt and anorthosite—which were remarkably different from those on the modern Earth. The water-rock interaction between these rocks and water provided a highly reducing environment and formed secondary minerals on the surface of the rocks that are important for producing metallo-enzymes for the emergence of primordial life. Previous studies suggested a correlation between the active site of metallo-enzymes and sulfide minerals based on the affinity of their structures, but they did not discuss the origin of metallic elements contained in these minerals which is critical to understanding where life began. We investigated secondary minerals formed through water-rock interactions of komatiite in a subaerial geyser system, then discussed the relationship between the active site of metallo-enzymes and secondary minerals. Instead of komatiite, we used serpentinite collected from the Hakuba Happo area, Nagano Prefecture in central-north Japan, which is thought to be a modern analog for the Hadean environment. We found several minor minerals, such as magnetite, chromite, pyrite and pentlandite in addition to serpentine minerals. Pentlandite has not been mentioned in previous studies as one of the candidates that could supply important metallic elements to build metallo-enzymes. It has been shown to be a catalyst for hydrogen generation possibly, because of structural similarity to the active site of hydrogenases. We consider the possibility that nickel-iron sulfide, pentlandite, could be important minerals for the origin of life. In addition, we estimated what kinds of minor minerals would be obtained from the water-rock interaction of these rocks using thermodynamic calculations. KREEP basalt contains a large amount of iron and it could be useful for producing metallo-enzymes, especially ferredoxins—electron transfer enzymes, which may have assisted in the emergence of life.
Similar content being viewed by others
Introduction
The existence of water is necessary, but that alone is not enough for the birth of life. Nitrogen and carbon were likely major components of primitive Earth’s atmosphere and are also major components of amino acids. On the other hand, important nutrients for life such as P and K and another 20 or more elements are derived from landmass. In other words, life did not emerge without the coexistence of the atmosphere, the ocean, and landmass. This is the concept of the “Habitable Trinity”, which was proposed by Dohm and Maruyama (2015). Extraterrestrial materials such as carbonaceous chondrites are other possible sources of prebiotic amino acids for early life on Earth (Pizzarello and Cronin 2000). If the Earth’s atmosphere were as reducing as in Miller’s experiments (1953), amino acid could be formed on Earth. However, recent theoretical studies suggest that Earth’s early atmosphere was slightly oxidizing (e.g. Kasting 1989) which makes amino acids synthesis difficult. Therefore, formation of semi-closed environments filled with reduced gases, such as H2, may have been important for forming prebiotic amino acids on early Earth. Although the amount of nutrients that contribute to the creation of all life forms is less than 5%, they play an important role because no life form can evolve and survive without rocks. Metallo-proteins play an especially important role in the evolution of life because the active sites of metallo-proteins have one or more transition metals—which assist in energy flow and provide important functions—that are indispensable for life.
Major essential bioelements—such as P, K, Fe, Ca, Mg—are contained in Hadean surface rocks such as komatiite, KREEP basalt, and anorthosite, and water-rock interaction provides these essential elements into the solution in various dissolved phases, along with important materials for the building blocks of biomolecules.
Among these important elements, transition metals are crucial for life that may allow electron flow in proteins. The act of moving an electron from a donor site to an acceptor site is a simple process in biological systems. Most electron transfers in metallo-proteins have a reduced transition metal-sulfur complex in their active site because the redox potential of sulfur is low. On the other hand, iron is one of the most abundant elements on Earth and likely existed as ferrous iron in Hadean reducing hydrothermal environments. It is important that water-rock interactions between Hadean rocks and water provide transition metal ions into solution in reducing hydrothermal environments.
As the temperature of the magma ocean gradually dropped, fractional crystallization occurred. During the magma differentiation, crystallization of olivine and pyroxene occurred at high temperatures of more than 1000 °C. Although these minerals are relatively slow to react with water at low temperature (<100 °C), the minerals could provide considerable amount of cations to the aqueous solution by reaction with the Hadean meteoric water in the presumed CO2-rich atmosphere (e.g. Neubeck et al. 2014). As a result of such reactions, several secondary minerals form on the surface of the minerals and dissolved metal ions are produced. These dissolved ions are important substrates and nutrients for life.
Therefore, many primitive metallo-proteins which may be related to the origin of life commonly contain [Fe4S4] clusters in their active sites. The idea that these iron sulfur clusters [Fe4S4] are important for life originated from iron sulfide (FeS) minerals, whose existence on the surface of the early Earth was proposed over 50 years ago (Eck and Dayhoff 1966). Russell and co-workers also suggested that polypeptides synthesized on early Earth incorporate [Fe4S4] iron sulfur cluster possessed by the greigite to assemble primitive metallo-proteins. They arrived at this conclusion because there are a lot of sulfide minerals such as greigite observed near deep ocean alkaline hydrothermal vents and greigite has an inverse spinel crystal structure similar to that of the [Fe4S4] cluster (Russell and Hall 1997; Russell and Martin 2004; Russell and Hall 2006). Recently, their research shows a correlation between the crystal structure of transition metal sulfide and oxide minerals, such as nickelian mackinawite, a nickel-bearing greigite, violarite and the active site of metallo-enzymes. They may be respectively compared with the active sites of Ni–Fe hydrogenase, carbon monoxide dehydrogenase (CODH), and acetyl coenzyme-A synthase (ACS) (Nitschke et al. 2013; Russell et al. 2014). However, they obviously did not mention that these minerals were found at the mounds of deep-ocean hydrothermal vents. They pointed out the structural similarity of transition metal sulfide/oxide minerals and the active sites of metallo-enzymes, but made no suggestion of a relationship with Hadean surface rocks, which may have provide metallo-enzymes and elements essential for life.
In this study, we focused on the water-rock interaction of Hadean surface rock, and the relationship with important secondary minerals and metallo-enzymes. First, we make detailed observations of serpentinite collected from the Hakuba Happo area, an environment that is a modern analog for the Hadean, as a water-rock interaction of komatiite (Suda et al. 2014). For the KREEP basalt, we use a whole-rock composition of a lunar sample—74,220, orange glass—which was collected by Apollo 17 at Shorty Crater (Meyer 2010). For anorthosite, we use remote sensing data collected from the moon’s surface by Kaguya (SELENE) (Ohtake et al. 2009). For KREEP basalt and anorthosite, we used thermodynamic calculations to evaluate the solution compositions and the possibility of transition metal minerals forming.
Rock Types Necessary for the Birthplace of Life
Rock Types in the Hadean Surface
In this section, we discuss what kind of rocks existed on the Hadean surface. A third of the modern Earth’s surface is covered by continental crust that is mainly composed of granitic rocks, which supplies 29 essential (trace) elements necessary for life (Maruyama et al. 2013). The rest of the Earth’s surface mainly consists of basaltic oceanic crust and is covered by the ocean. Although granitic rocks possess well-balanced essential elements for life, basaltic rocks contain low amounts of P and which is an essential nutrient for life.
There are a few remnants of Hadean geological records (from 4.6 to 4.0 Ga) on the surface of the Earth: rocks from the Nuvvuagittuq greenstone belt in northern Quebec, Canada (O’Neil et al. 2008), and the Hadean detrital zircons from the Jack Hills (Wilde et al. 2001; Valley et al. 2014), the Mount Narryer (Froude et al. 1983), the Acasta gneiss (Iizuka et al. 2006), and the Barberton Greenstone Belt (Byerly et al. 2018). The mere survival of these rocks and minerals over time indicates that regions akin to continental crust existed by 4.4 Ga and possibly as early as 4 5 Ga (Sleep 2010). Many scientists believe that the first crust was mantle-derived basaltic rock, and more Mg-rich and higher temperature komatiites (Sleep 2010; Arndt and Nisbet 2012). On the other hand, Hadean detrital zircons contain high uranium concentrations compared with zircons derived from mafic sources (Crowley et al. 2005). It is suggested that their source magma is relatively felsic magma. In addition, mineralogical and morphological differences between Jack Hills and Mount Narryer zircons suggest that Earth’s crust was heterogeneous by 4 2 Ga (Crowley et al. 2005). If the Hadean crust repeatedly underwent internal partial melting, it may have produced basaltic rocks enriched in KREEPy elements that could have crystallized the Hadean zircons (Kemp et al. 2010; Arndt and Nisbet 2012). Results of theoretical calculations suggest that if the Hadean Earth was dry and rich in CaO and Al2O3, anorthosite could be another candidate (Santosh et al. 2017). Although information regarding Hadean surface rock composition is still limited, we consider Hadean surface could have consisted of komatiite, KREEP basalt and anorthosite.
The Early Earth Environment and the Birthplace of Protolife
Several environments have been proposed as the birthplace of life: deep-sea hydrothermal vents at mid-oceanic ridges (Baross and Hoffman 1985; Russell and Hall 1997 etc.), hydrothermal-sedimentary micro-reactor environments (Westall et al. 2018), subaerial hydrothermal geysers (Mulkidjanian et al. 2012; Damer and Deamer 2015 etc.), and subaerial serpentinite-hosted hydrothermal nuclear geyser systems (Ebisuzaki and Maruyama 2017) among others. Among these proposed environments for the birthplace of life, serpentinite-hosted subaerial hydrothermal systems, which form highly reducing environments with hydrogen generation due to water-rock interaction, are thought to be plausible environments for proto life. In addition, the accumulation of high concentrations of hydrogen gas in semi-closed spaces of geyser systems and hydration dehydration cycles for polymerization of amino acids may have been necessary for the origin of life. Although there are several proposals for the origin of life, we consider that the subaerial serpentinite-hosted hydrothermal nuclear geyser systems plausible due to the availability of an energy source, material cycling, and accumulation of reducing gases; all of which may be necessary for the creation of life (Ebisuzaki and Maruyama 2017).
Metallo-Enzymes of Primordial Life
Proteins are polymers of amino acids formed from light elements such as C, N, H, O, and S. However, in order for them to function as life-forming molecules, continuous chemical reaction in a non-equilibrium state needs to occur. A mechanism is needed to maintain a continuous flow of electrons within cells (Sato et al. 2019). Thus, simple proteins formed from a combination of nine different amino acids that merged with transition metals could provide a feasible mechanism of continuous chemical reaction using free electron transfer. Metallo-proteins play many different roles in cells, such as storage, electron transfer, and signal transduction. What kinds of metallo-enzymes did primordial life possess?
Weiss et al. (2016) took a phylogenetic approach to identify genes that can illuminate the biology of the last universal common ancestor (LUCA). They considered two simple criteria for LUCA proteins: (1) the protein should be present in at least two higher taxa of bacteria and archaea, respectively, and (2) its tree should recover bacterial and archaeal monophyly (Weiss et al. 2016). Their functions, properties and prosthetic groups depict LUCA as anaerobic, CO2-fixing, H2-dependent with a Wood–Ljungdahl pathway, N2-fixing and thermophilic (Weiss et al. 2016). Therefore, we estimate that metallo-enzymes in primordial life were low redox potential and had relatively lower molecular weight than modern proteins. The following are major metallo-enzymes of primordial life: ferredoxin, hydrogenase, and Acetyl-CoA Synthase/Carbon Monoxide Dehydrogenase (ACS/CODH) complex.
Ferredoxins
Ferredoxins are a class of iron-sulfur metallo-proteins. Ferredoxins from anaerobic bacteria have a very simple composition and their active sites contain two [Fe4S4] iron-sulfur clusters (Fig. 1). These clusters act as electron carriers in various biochemical processes, such as carbon metabolism, nitrogen fixation, photosynthesis and others (Eck and Dayhoff 1966). They are relatively small and are the simplest proteins with molecular weights of 6000 to 12,000; they contain 2–8 atoms of iron, equivalent amounts of sulfur, and transfer electrons at low redox potentials (−300 to −500 mV) (Hall et al. 1971). They are considered to be the first short polypeptide chain formed from 26 amino acids comprising only nine different amino acids, which are easily produced in simulated primeval conditions (Hall et al. 1971). It may have been possible for primitive organisms to uptake amino acids from the environment to synthesize this simple protein and combine it with the widely dispersed mineral, FeS, as the active site (Eck and Dayhoff 1966; Hall et al. 1971).
Ferredoxins are small, simple proteins of fifty-five amino acids and molecular weight 6000 with a primitive polypeptide structure that act as electron carriers. Structure data taken from PDB entry 2FND. Orange and yellow balls are iron and sulfur, respectively. Crystal structure of [Fe4S4] iron- sulfur cluster
Hydrogenase
Hydrogenases catalyze one of the simplest molecular reactions: the conversion of dihydrogen into protons and electrons, and the reverse reaction.
Hydrogenases provide energy for organisms from molecular hydrogen and proton reduction coupled to electron donors, such as ferredoxins, to maintain a reducing condition inside the cell. Hydrogenases work with other small proteins or low molecular compounds, such as ferredoxin, cytochrome c3, and cytochrome c6. Hydrogenases are widespread in nature: they occur in bacteria, archaea, and some eukarya (Vignais and Billoud 2007). Hydrogenases can be classified by the metal ion composition of their active sites in [NiFe], [FeFe], and [Fe] hydrogenases (Vignais and Billoud 2007; Fontecilla-Camps et al. 2009). Although [Fe] hydrogenases, which only present in methanogenic archaea, were initially referred to as “metal-free” hydrogenases, Lyon et al. (2004) revealed that their active site contains a single iron atom.
Most known hydrogenases are iron-sulfur proteins with two metal atoms at their active site: a Ni and an Fe atom (in [NiFe]-hydrogenases) or two Fe atoms (in [FeFe]-hydrogenases). There are structural similarities between [NiFe]- and [FeFe]-hydrogenases (Fig. 2). Both types have metals in their active site and electron transport chains (via iron−sulfur centers), as well as pathways for dihydrogen and the H+ transfer (Lubitz et al. 2014). A characteristic feature of the [NiFe] and [FeFe] hydrogenases is that the iron atoms have small inorganic ligands (CO and CN−). [NiFe] hydrogenases are often more active in H2 oxidation and the [FeFe] hydrogenases in the production of molecular hydrogen (Lubitz et al. 2014).
Crystal structures of [FeFe]- and [NiFe]- hydrogenases and their active centers (Lubitz et al. 2014). Hydrogenases catalyze one of the simplest molecular reactions, the conversion of dihydrogen into protons and electrons and the reverse reaction. They can be classified according to the metal ion composition of their active sites in [NiFe]-, [FeFe]-, and [Fe]- hydrogenases
ACS/CODH Complex
Ni-dependent Acetyl-CoA synthase (ACS) and CO dehydrogenase (CODH) constitute the central enzyme complex of the Wood-Ljungdahl pathway, one of the primitive CO2 fixing pathway, which forms acetyl-CoA (Ljungdhal 1986; Volbeda et al. 2009). The ACS/CODH complex requires strictly anoxic conditions to function. The structure of this enzyme has been determined by the structural analysis technology of complicated proteins developed since 2000. As shown in Fig. 3, the ACS/CODH complex consists of four subunits: two CODH subunits in the center and one ACS subunit on both ends, respectively (Darnault et al. 2003; Matsumoto 2009). ACS dimers contain two A-clusters, and CODH dimers contain five metal-sulfur clusters of three types, called B-, C-, and D-clusters. A-clusters consist of Ni-Ni-[Fe4S4] (Svetlitchnyi et al. 2004) (Fig. 3b). B-clusters and D-clusters are the [Fe4S4] type. C-clusters consist of one Ni, four Fe, and five sulfur atoms (Fig. 3c). Electrons produced by the redox reaction in the C-cluster are delivered to proteins through the B- and D- clusters.
a Crystal structure of ACS/CODH complex (Darnault et al. 2003). ACS forms the bifunctional enzyme with carbon monoxide dehydrogenase (CODH), which converts carbon dioxide into carbon monoxide, Acetyl-CoA Synthase/Carbon Monoxide Dehydrogenase (ACS/CODH). The structure of the CODH/ACS enzyme consists of the CODH enzyme as a dimer at the center with two ACS subunits on each side (Ragsdale 2004). b Structure of A-cluster (Svetlitchnyi et al. 2004). c Structure of C-cluster (Dobbek et al. 2001)
Water-Rock Interaction of Hadean Surface Rocks and Secondary Minerals Produced
Ultramafic Rock and Secondary Minerals
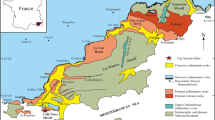
Serpentinite-hosted hydrothermal systems have drawn attention as hydrogen-producing Hadean analog environments for early life. In modern surface environments, serpentinite-hosted hydrothermal systems are distributed in oceanic tectonic plate boundaries, such as Lost City, and on lands ultramafic rock bodies. Compared with oceanic hydrothermal systems, there are few terrestrial serpentinite-hosted hydrothermal systems on modern Earth, these include The Cedars, the Samail Ophiolite, the Voltri Massif and Hakuba Happo. Hakuba Happo is a terrestrial serpentinite-hosted hydrothermal system with highly reducing (pH > 11) and high H2 concentration (664 μmol/L for Happo #1 well and 151 μmol/L for Happo #3 well) hot springs (Suda et al. 2014).
Recent metagenomic analyses of bacterial community in these serpentinite-hosted hydrothermal system sites reveal that ancient type microbes including members of candidate division OD1 inhabit these locations (Brazelton et al. 2010; Suzuki et al. 2017; Brazelton et al. 2017; Rempfert et al. 2017). OD1 have very small size genome (<1 Mbp) with severely reduced metabolic capabilities, and have attracted attention as one of the most primitive organisms on the present Earth (Sato et al. 2019). In this study, we use serpentinite rock samples collected from the host rock of Hakuba Happo hot spring, where a modern hydrogen-generation field exists due to serpentinization of ultramafic rocks (Fig. 4). We observed thin sections of these samples, using a microscope and SEM-EDS, and we discuss the relationship between secondary sulfide minerals and metallo-proteins of primordial life.
a Geologic map of the Hakuba area (modified from AIST 2015). The red dots are locations of sample collection. b The schematic cross-section showing the Hakuba-Happo hydrothermal system. Approximately 700 m depth drilling holes are located on the largest serpentinite block, and the hot water underground is pumped up from them. Hakuba OD1 was found in the hot spring water deep underground
Geological Setting of Hakuba Happo Area
The Hakuba Happo serpentinite rock is located in the northeastern part of the Hida Marginal Tectonic Belt on Honshu Island in central Japan. In the Hakuba area the biggest serpentinite rock body mainly consists of danite. Peridotite complexes suffer from strong shear deformation and about 80% of them are affected by serpentinization, through an approximately 10 km × 10 km sized area of boudinage danite rocks remain fresh. From several m to cm scale danite bourdins are observed in the outcrop. The structure of the shear plane is parallel to the crystalline schist of the northwestern margin. Along with recrystallization of ultramafic rock, the combination of secondary minerals including tremolite is divided into two metamorphic zones (Nakamizu et al. 1989). The eastern side of the solid line (tremolite zone) is a low-temperature area where we collected samples.
Location X shows the position of a 700 m deep two drilling wells for the hot spring. Uncultured microbes from candidate phylum OD1, Hakuba OD1, were found in these wells (Sato et al. 2019; Nakai et al. in prep).
The Hakuba peridotite block is a part of an oceanic plate which was emplaced on the proto-Japan arc around 600 Ma (before deep-sea oxygen levels increased). After the block was trapped by the Pacific orogenic belt, continuous water circulation caused a reaction with the rock body and produced hydrogen to maintain a highly reducing environment. From the geological aspect, Hakuba-Happo has preserved the most reducing conditions among known terrestrial serpentinite-hosted hydrothermal systems (Sato et al. 2019).
Detailed Observation of Serpentinite
Two samples of serpentinite, S2 and S3A, show different levels of serpentinization. Sample S2 remains as scale-shaped olivine relicts between the mesh-like structures of serpentine minerals. Relicts of olivines account for approximately 35% of the total. In contrast, most parts of sample S3A consist of serpentine and a small amount of olivine relicts are observed, suggesting 95% of this sample is affected by serpentinization. The modal abundances of antigolite, lizardite, and chrysotile in serpentine are about 90%, 10%, and 0%, respectively, suggesting that these sample rocks are fresh.
Two relatively large and clear olivine (forsterite) relicts (Fo = 9x) are seen in the thin sections. Several relicts of indistinct shape are observed. From the texture, it is obvious that serpentinization of olivine provides secondary minerals such as serpentine, clinochlore, and magnetite (Figs. 5, 6). SEM-EDS observation reveals that the enlarged opaque minerals consist of two different mineral types, magnetite and nickel iron sulfides, and pentlandite ((Fe, Ni)9S8). Most of the magnetite grains are associated with pentlandite. A majority of the Ni in the pentlandite originated from olivine.
(Upper photos) Microphotos (left side is cross polarized photo, right side photo is open nicole) of thin section of sample S2. Olivine affected by water-rock reaction to form serpentine and opaque mineral. (lower photos) Enlarged SE image of opaque minerals. They consist of magnetite and pentlandite exsolution lamellae. Light gray area is pentlandite and dark gray area is magnetite
(Upper photos) Microphotos (left side is open nicole photo, right side is cross polarized photo) of thin section of sample S3A. Large forsterite relict observed. (lower photos) Enlarged SE image of an opaque mineral. It consists of magnetite and pentlandite exsolution lamellae. Light gray area is pentlandite and dark gray area is magnetite
Thermodynamic Calculation of KREEP Basalt, Anorthosite, and Primitive Upper Mantle Peridotite
The Hadean surface rocks are considered to be destroyed by the heavy meteorites bombardment (e.g. Bottke et al. 2012; Kamber 2015) and most Hadean rocks have been eliminated by stagnant-lid type convection or plate tectonics (e.g. Harrison et al. 2005; Zahnle et al. 2007; Korenaga 2007). And no modern analog environments exist for KREEP basalt and anorthosite. However, similar types of Hadean rocks are preserved on the surface of the moon because no material cycle has occurred there since the solidification of the lunar magma ocean.
For KREEP basalt, we used whole-rock compositions of lunar samples, collected by the Apollo missions. For anorthosite, we use a composition of the purest anorthosite based on the SELENE observation. Then we use thermodynamic calculations to estimate what kinds of secondary minerals were made by water-rock interaction for individual rocks. In addition, we also use model composition of primitive upper mantle peridotite for comparison of solution compositions obtained from thermodynamic calculations for each rock.
We used whole-rock composition of Fe-Ti rich mare basalt 74,220 (orange glass) (Fig. 7a) for KREEP basalt (Rhodes et al. 1974; Wänke et al. 1973). Ordinary KREEP basalts, collected at the surface of the moon, generally have been affected by meteorite impacts. The sample, 74,220 orange glass, is considered to have originated from high-Ti magma which is likely developed from lunar magma ocean (Hughes et al. 1989). According to Meyer (2010), the sample 74,220 basaltic glass was erupted from about 200 km depth which is presumed to preserve the composition of KREEP basalt just after the solidification of lunar magma ocean. We calculate a CIPW norm to determine constituent minerals from 74,220 basaltic glasses (Fig. 7b). For anorthosite, we refer to the remote sensing data collected from the moon’s surface by Kaguya (Ohtake et al. 2009), and we assume Purest Anorthosite (PAN, 100% Ca-rich Plagioclase: CaAl2Si2O8). For the model composition of the upper mantle peridotite, we refer to McDonough and Sun (1995). We use thermodynamic calculation assuming 100 g of rock in the presence of 500 g water under conditions of 1 atm, and temperatures from 0 to 100 °C. Calculation results are shown in Fig. 7b. We used the GEM-Selektor v.3 (GEMS3) program (Kulik et al. 2013), which provides concentrations of the dissolved species in equilibrium from the chemical composition of given rocks and water using the Gibbs energy minimization method. We used SUPCRT92 for the thermodynamic dataset (Johnson et al. 1992).
a Whole-rock composition of the lunar KREEP basalt (74,220 orange glass) (Rhodes et al. 1974; Wänke et al. 1973). The mineral compositions of the 74,220 were estimated using norm calculations. b Using thermodynamic calculations, we estimate minerals obtained from water-rock reaction of 100 g of KREEP basalt (74,220 orange glass) collected from the moon’s surface, and reaction of 100 g of PAN with 500 g of water, respectively
Major secondary minerals estimated from calculation of water-rock reactions of KREEP basalt are hematite, titanite, and talc. Besides these, a small amount of Al-Ca rich clay minerals such as kaolinite, and laumontite are obtained. Calculations of water-rock interactions with PAN result in secondary minerals including a combination of hydroxide minerals such as gibbsite, prehnite, and kaolinite. From this calculation, we can only obtain major secondary minerals. For the minor secondary minerals, which are important for formation of metallo-proteins, we need to evaluate remaining ion compositions in solutions.
Figure 8 shows the concentration of representative ions in solutions reacted with KREEP basalt, PAN, and PUM (primitive upper mantle; as komatiite). Ion concentrations reflect whole-rock compositions of each rock. The PO43− concentration of KREEP basalt is more than five times higher than that of PUM. The pH of the PUM rock solution is highest, followed by that of PAN and KREEP basalt. As the difference is only 0.5 pH units, it suggests that the alkaline solution environments can also be formed by water-rock interaction of PAN or KREEP basalt.
Discussion
Relationship between Secondary Minerals Formed from Serpentinization and Active Site of the Metallo-Proteins
Secondary Minerals Formed from Serpentinization
We summarize what kind of chemical reactions occurred and what kind of secondary minerals were constructed during water-rock interactions between ultramafic rocks and water. Major components of komatiite are olivine (60% in mode count) and pyroxene. Olivine is a magnesium iron silicate with the formula (Mg2+, Fe2+)2SiO4. The magnesium to iron ratio varies between the two endmembers: forsterite (Mg-endmember: Mg2SiO4) and fayalite (Fe-endmember: Fe2SiO4). Fayalite reacts with water-generated hydrogen to form Fe-serpentinite (reaction 1).
This disequilibrium reaction partially produces a highly reducing environment (Frost 1985). At 500 bar, when the temperature is higher than 350 °C, the combination of water and olivine is thermodynamically stable. If the temperature is lower than 350 °C, serpentinite and brucite become stable instead of olivine.
Reaction 1 represents serpentinization, which produces serpentine as a secondary mineral during water-rock interaction. After serpentinite has emerged from the water-rock interaction, as long as the water-rock reaction persists, hydrogen is continuously provided and the highly reducing environment is maintained (reaction 2, 3), which is very crucial for the formation process of amino acids (Sakata et al. 2014).
The production of magnetite from reactions 1 to 3 is consistent with the thin section observation in this study. Besides iron, olivine also contains trace amounts of transition metals such as nickel, manganese, and titanium. During serpentinization, a highly reducing environment produced by hydrogen generation and secondary minor opaque minerals are formed, including a combination of magnetite and pentlandite or other nickel-containing sulfides, such as healzewoodite and vaesite (Nozaka 2012).
Crystal Structure of Active Site of Metallo-Proteins and Pentlandite
We focused on pentlandite (Ni4.5Fe4.5S8) in this study because we found it as a secondary minor mineral from Hakuba Happo serpentinite. Pentlandite has not been mentioned in previous studies of origin of life regarding the active sites of metallo-enzymes.
Pentlandite consists of iron, nickel and sulfur. The nickel and iron share the same positions within the crystal bridged by sulfur, and the mineral has high electric conductivity. Its structural features closely resemble the active site of [Fe-Ni] and [Fe-Fe] hydrogenases. The intermetallic distance of pentlandite is about 2. 51 Å, and that of [Fe-Ni] and [Fe-Fe] hydrogenase is dFeNi ~ = 2. 57 Å and dFeNi ~ = 2. 57 Å, respectively (Nicolet et al. 1999; Ogata et al. 2015). From these structural and electro-chemical features, pentlandite is focused on as a sustainable, efficient electro-catalyst (Konkena et al. 2016).
It is interesting that the structural features of secondary minerals obtained from water-rock interactions of Hadean surface rocks closely resemble the active site of metallo-enzymes of primordial life, such as hydrogenase and the ACS/CODH complex. This suggests that the transition metals found in the active sites of metallo-enzymes were provided by secondary minerals from Hadean rocks that existed in the birthplace of life. From the metagenome analysis of Hakuba Happo hot spring samples, suggests that one of the most primitive candidate division OD1, Hakuba OD1, or associated microorganisms may also contain this pathway (Nakai et al. in prep).
KREEP Basalt and Pentlandite
From our investigation of serpentinite rock, water-rock interactions provide the secondary sulfide mineral pentlandite, which may have an important role in composing active sites of hydrogenases and the ACS/CODH complex. However, the amount of transition metals in komatiite is quite small. Sulfur, which is necessary for iron-sulfur clusters, is also only found in trace amounts. In contrast, the whole-rock composition of KREEP basalt (Fig. 7a) contains many kinds of elements essential for life, including transition metals, which are necessary for metallo-enzymes. It contains sulfur (0.07 wt%) as a major component, and a large amount of iron (as FeO 22.04 wt%; Fig. 7a) (Wänke et al. 1973; Rhodes et al. 1974). In comparison with the iron contents in the Archean basalt (at most 17.2 wt%) (Ohta et al. 1996), the amount found in KREEP basalt is higher than that of Archean basalt. Secondary minerals obtained from water-rock interactions of KREEP basalt could form a larger amount of FeS clusters than that of the Archean basalt for useful metallo-proteins.
KREEP basalt contains other major components such as magnesium, calcium, and phosphorus. Some other minor components, such as zinc, cobalt, and nickel are also important for metallo-proteins active sites. There is no remarkable difference between the nickel content of modern basalt (both island arc basalt and MORB: several 10 to 100 ppm, Kawabe 1974) and that of KREEP basalt. On the other hand, the nickel contents of Archean komatiites and basalts range from 1000 to 2000 ppm (Nesbitt et al. 1979; Sossi et al. 2016), higher than that of modern basalt. Pentlandite is a major mineral obtained from mafic nickel deposits. One of the typical examples is the Noril’sk-Talnakh nickel deposit, which formed 250 million years ago (Arndt et al. 2003). In this case, pentlandite is crystallized from magma as a primary mineral, and not from water-rock interactions. However, it is suggested that nickel sulfur minerals could be concentrated when KREEP basalts were formed from Hadean mafic magma.
Environmental Conditions where the Metallo-Proteins Were Formed
In this section, we discuss the environmental conditions where and when the simple metallo-proteins, such as ferrodoxins, formed. Proteins are biomolecules, consisting of one or more long chains of amino acid. As was previously mentioned, ferredoxins are small metallo-proteins that transfer electrons at low redox potentials. Unlike other electron transfer proteins, the active sites of ferredoxins contain only iron and inorganic sulfur (Hall et al. 1971). General biosynthetic principles seem to underlie the in-vivo synthesis of Fe–S clusters and their assembly into apoproteins (Lill 2009). This process is known to occur under anaerobic conditions (Malkin and Rabinowitz 1966; Hall et al. 1971).
Weiss et al. (2016) depict LUCA as inhabiting an anaerobic, iron-rich, H2-dependent environment. They also identified the presence of reverse gyrase, an enzyme specific for hyperthermophiles, in LUCA. This suggests that the temperature of habitat of LUCA was hotter than 70 °C.
Therefore, the primordial metallo-proteins also likely developed in a relatively hot (more than 70 °C), highly alkaline environment that was rich in hydrogen provided by serpentinite-hosted hydrothermal systems.
Evaluation of Solution Environment
We calculated the major ion concentrations of the water-rock interaction of KREEP basalt, PAN and PUM using thermodynamic calculation programs. The results reflect the composition difference between these three rocks. As the composition of KREEP basalt is rich in K, REE (rare earth elements) and P, its natural PO43− ion concentration is remarkably higher than the solution of other rocks. On the other hand, no distinct difference is seen between other major ions such as Mg2+, Ca2+, Fe2+. Recently, researchers proved that alkaline conditions are favorable for prebiotic oligomerization (Sakata et al. 2014; Rodriguez-Garcia et al. 2015). Subaerial serpentinite-hosted hydrothermal systems, such as Hakuba and the Cedars where OD1 lives, provide a highly alkaline environment of pH 10–11 (Suda et al. 2014; Suzuki et al. 2013, 2017). It is believed that the highly alkaline conditions in a serpentinite-hosted hydrothermal system are due to the dissolution of Mg(OH)2. The thermodynamic calculations in this study show that water-rock interactions of KREEP basalt and PAN produce highly alkaline solutions; it is not as high as PUM, but the difference is in the order of 0.5 pH units. This is due to the dissolution of CaO contents in rocks providing an alkaline solution:
Therefore, a high alkaline environment can also be provided by PAN and KREEP basalt, and not only komatiite.
Using thermodynamic calculations, we evaluated whether Ni sulfide minerals could have existed in a stable condition in the solution environment. Figure 9 shows stable areas of Ni and Fe sulfide minerals in fS2-fO2 and pH-fO2 systems at 80 °C (Fig. 9a, b). These figures suggest that, in relatively low fO2 conditions fO2 < −60, Ni sulfide minerals can be stable even though the hydrothermal system has a relatively low temperature (80 °C). On the other hand, under a high fO2 fugacity or acidic conditions under which Fe sulfate forms, Ni sulfide minerals will dissolve. This means that, if sulfide minerals were involved in the formation of primordial metallo-proteins, the environment where life emerged should be at low fO2 and high pH.
fS2-fO2 and pH-fO2 diagrams at 80 °C, in the Ni-S-O-H and Fe-S-O-H systems. The Geochemist’s Workbench with its built-in dataset “thermo.com.V8.R6+” was used for the calculations. Solid lines and dotted lines represent phase boundary in Ni and Fe minerals, respectively. Pentlandite does not appear in these diagrams because of the metastability of its Ni end-member. However, we estimate the stability field of pentlandite to be in the heazlewoodite + magnetite stability field
Conclusions
This work presents the formation of metallo-enzymes in primordial life from the secondary minerals produced by water-rock interaction of Hadean surface rocks: komatiite, KREEP basalt and anorthosite. Water running into a subaerial geyser system interacts with olivine in the komatiite wall and produces hydrogen gas. Accumulated hydrogen gas forms a highly reducing environment in the geyser system. In addition to the ultra-reducing water environment produced in the geyser system, secondary minerals were formed on the surface of Hadean rock. In this study, we analyzed serpentinite rocks from one of the modern analogs for the Hadean, Hakuba Happo. The serpentinite rocks were formed by hydrothermal water-rock interaction of ultramafic rock. We made detailed microscopic observations of its secondary minerals. In addition to iron sulfide minerals such as pyrite, which has already been indicated in previous studies, we found iron-nickel sulfide, and pentlandite (Ni4.5Fe4.5S8) associated with magnetite. As the crystal structure of pentlandite closely resembles the active site of [Fe-Ni] hydrogenase and ACS/CODH complex, we pointed out that pentlandite could be an important candidate mineral in metallo-enzyme formation for primordial life. The ACS/CODH complex is especially important as one of the crucial metallo-enzymes for the early stages of CO2 fixation via the Wood-Ljungdahl pathway. However, the amount of iron-nickel sulfide in serpentinite is very low, and very few phosphorus ions are supplied into the solution by water-rock interaction of komatiite.
We used thermodynamic calculations of water-rock interaction of KREEP basalt and anorthosite using whole-rock analysis of orange glass samples discovered at Shorty Crater, Apollo 17 (74220) and PAN. With respect to the trace amount of iron nickel sulfide contained in serpentinite, the whole-rock composition of KREEP basalt contains a large amount of iron and necessary components to build metallo-enzymes of primordial life. In addition, water-rock interactions of KREEP basalt produce a PO43− ion-rich solution, which is 5 orders of magnitude more concentrated than that derived from komatiite. We conclude that KREEP basalt, believed to have existed on the Hadean surface, could provide minerals such as iron and iron-nickel sulfides that are necessary for the emergence of metallo-enzymes. The whole-rock composition of KREEP basalt also suggests it can supply phosphorus and other elements necessary for life.
We present the importance of the existence of Hadean surface rocks and subaerial serpentinite-hosted hydrothermal geyser systems, and the water-rock interaction of these rocks with water. It produces highly reducing environments in the semi-closed space of a geyser, and highly alkaline solutions that contain various kinds of cations provided by rocks. In such highly reducing environments provided by Hadean rocks, metal sulfide minerals could be incorporated into the active sites of metallo-proteins—such as ferredoxins, hydrogenases and the ACS/CODH complex—in the building block of biomolecules.
References
Arndt NT, Nisbet EG (2012) Processes on the young earth and the habitats of early life. Annu Rev Earth Planet Sci 40:521–549
Arndt NT, Czamanske GK, Walker RJ, Chauvel C, Fedorenko VA (2003) Geochemistry and origin of the intrusive hosts of the Noril’sk-Talnakh cu-Ni-PGE sulfide deposits. Econ Geol 98:495–515
Baross JA, Hoffman SE (1985) Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig Life Evol Biosph 15:327–345
Bottke WF, Vokrouhlický D, Minton D, Nesvorný D, Morbidelli A, Brasser R, Simonson B, Levison HF (2012) An Archaean heavy bombardment from a destabilized extension of the asteroid belt. Nature 485:78–81
Brazelton WJ, Ludwig KA, Sogin ML, Andreishcheva EN, Kelley DS, Shen CC, Edwards RL, Baross JA (2010) Archaea and bacteria with surprising microdiversity show shifts in dominance over 1,000-year time scales in hydrothermal chimneys. Proc Natl Acad Sci 107:1612–1617
Brazelton WJ, Thornton CN, Hyer A, Twing KI, Longino AA, Lang SQ, Lilley MD, Früh-Green GL, Schrenk MO (2017) Metagenomic identification of active methanogens and methanotrophs in serpentinite springs of the Voltri massif, Italy. PeerJ 5:e2945
Byerly BL, Lowe DR, Drabon N, Coble MA, Burns DH, Byerly GR (2018) Hadean zircon from a 3.3 Ga sandstone, Barberton greenstone belt, South Africa. Geology 46:967–970
Crowley JL, Myers JS, Sylvester PJ, Cox RA (2005) Detrital zircon from the Jack Hills and mount Narryer, Western Australia: evidence for diverse> 4.0 Ga source rocks. J Geol 113:239–263
Damer B, Deamer D (2015) Coupled phases and combinatorial selection in fluctuating hydrothermal pools: a scenario to guide experimental approaches to the origin of cellular life. Life 5:872–887
Darnault C, Volbeda A, Kim EJ, Legrand P, Vernède X, Lindahl PA, Fontecilla-Camps JC (2003) Ni-Zn-[Fe4-S4] and Ni-Ni-[Fe4-S4] clusters in closed and open α subunits of acetyl-CoA synthase/carbon monooxide dehydrogenase. Nat Struct Biol 10:271–279
Dobbek H, Svetlitchnyi V, Gremer L, Huber R, Meyer O (2001) Crystal structure of a carbon monoxide dehydrogenase reveals a [Ni-4Fe-5S] cluster. Science 293:1281–1285
Dohm JM, Maruyama S (2015) Habitable trinity. Geosci Front 6:95–101
Ebisuzaki T, Maruyama S (2017) Nuclear geyser model of the origin of life: driving force to promote the synthesis of building blocks of life. Geosci Front 8:275–298
Eck RV, Dayhoff MO (1966) Evolution of the structure of ferredoxin based on living relics of primitive amino acid sequences. Science 152:363–366
Fontecilla-Camps JC, Amara P, Cavazza C, Nicolet Y, Volbeda A (2009) Structure–function relationships of anaerobic gas-processing metalloenzymes. Nature 460:814–822
Frost BR (1985) On the stability of sulfides, oxides, and native metals in serpentinite. J Petrol 26:31–63
Froude DO, Ireland TR, Kinny PD, Williams IS, Compston W, Williams IR, Myers JS (1983) Ion microprobe identification of 4,100–4,200 Myr-old terrestrial zircons. Nature 304:616–618
Geological Survey of Japan, AIST (ed.) (2015) Seamless digital geological map of Japan 1: 200,000. May 29, 2015 version. Geological Survey of Japan, National Institute of Advanced Industrial Science and Technology.
Hall DO, Cammack R, Rao KK (1971) Role for ferredoxins in the origin of life and biological evolution. Nature 233:136–138
Harrison TM, Blichert-Toft J, Müller W, Albarede F, Holden P, Mojzsis SJ (2005) Heterogeneous hadean hafnium: evidence of continental crust at 4.4 to 4.5 Ga. Science 310:1947–1950
Hughes SS, Delano JW, Schmitt RA (1989) Petrogenetic modeling of 74220 high-Ti orange volcanic glasses and the Apollo 11 and 17 high-Ti mare basalts. In Lunar and Planetary Science Conference Proceedings 19:175–188
Iizuka T, Horie K, Komiya T, Maruyama S, Hirata T, Hidaka H, Windley BF (2006) 4.2 Ga zircon xenocryst in an Acasta gneiss from northwestern Canada: evidence for early continental crust. Geology 34:245–248
Johnson JW, Oelkers EH, Helgeson HC (1992) SUPCRT92: a software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species, and reactions from 1 to 5000 bar and 0 to 1000°C. Comput Geosci 18:899–947
Kamber BS (2015) The evolving nature of terrestrial crust from the hadean, through the Archaean, into the Proterozoic. Precambrian Res 258:48–82
Kasting JF (1989) Long-term stability of the Earth's climate. Glob Planet Chang 1:83–95
Kawabe I (1974) Transition metal contents of Paleozoic geosynclinal basalts in Southwest Japan and their geological significance. J Geol Sot Jpn 80:539–554 (in Japanese with English abstract)
Kemp AIS, Wilde SA, Hawkesworth CJ, Coath DD, Nemchin A, Pidgeon RT, Vervoort JD, DuFrane SA (2010) Hadean crustal evolution revisited: new constraints from Pb-Hf isotope systematics of the Jack Hills zircons. Earth Planet Sci Lett 296:45–56
Konkena B, Junge Puring K, Sinev I, Piontek S, Khavryuchenko O, Dürholt JP, Schmid R, Tüysüz H, Muhler M, Schuhmann W, Apfel UP (2016) Pentlandite rocks as sustainable and stable efficient electrocatalysts for hydrogen generation. Nat Commun 7:12269–12276
Korenaga J (2007) Thermal cracking and the deep hydration of oceanic lithosphere: a key to the generation of plate tectonics. J Geophys Res 112:B05408
Kulik DA, Wagner T, Dmytrieva SV, Kosakowski G, Hingerl FF, Chudnenko KV, Berner UR (2013) GEM-Selektor geochemical modeling package: revised algorithm and GEMS3K numerical kernel for coupled simulation codes. Comput Geosci 17:1–24
Lill R (2009) Function and biogenesis of iron–Sulphur proteins. Nature 460:831–838
Ljungdhal LG (1986) The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol 40:415–450
Lubitz W, Ogata H, Rüdiger O, Reijerse E (2014) Hydrogenases. Chem Rev 114:4081–4148
Lyon EJ, Shima S, Boecher R, Thauer RK, Grevels FW, Bill E, Albracht SP (2004) Carbon monoxide as an intrinsic ligand to iron in the active site of the iron− sulfur-cluster-free hydrogenase H2-forming methylenetetrahydromethanopterin dehydrogenase as revealed by infrared spectroscopy. J Am Chem Soc 126:14239–14248
Malkin R, Rabinowitz JC (1966) The reconstitution of clostridial ferredoxin. Biochem Biophys Res Commun 23:822–827
Maruyama S, Ikoma M, Genda H, Hirose K, Yokoyama T, Santosh M (2013) The naked planet earth: Most essential pre-requisite for the origin and evolution of life. Geosci Front 4:141–165
Matsumoto T (2009) Acetyl CoA synthase, a key player of carbon fixation in nature. Bulletin of Japan Society of Coordination Chemistry 54:38–51
McDonough WF, Sun SS (1995) The composition of the earth. Chem Geol 120:223–253
Meyer, C., 2010. The lunar sample compendium. https://curator.jsc.nasa.gov/lunar/lsc/74220.pdf. Accessed 27 Nov 2017
Miller SL (1953) A production of amino acids under possible primitive earth conditions. Science 117:528–529
Mulkidjanian AY, Bychkov AY, Dibrova DV, Galperin MY, Koonin EV (2012) Origin of first cells at terrestrial, anoxic geothermal fields. Proc Natl Acad Sci 109:E821–E830
Nakamizu M, Okada M, Yamazaki T, Komatsu M (1989) Metamorphic rocks in the Omi-Renge serpentinite mélange, Hida marginal Tectonic Belt, Central Japan. Mem Geol Soc Jpn 33:21–35 (in Japanese with English abstract)
Nesbitt RW, Sun SS, Purvis AC (1979) Komatiites; geochemistry and genesis. Can Mineral 17:165–186
Neubeck A, Duc NT, Hellevang H, Oze C, Bastviken D, Bacsik Z, Holm NG (2014) Olivine alteration and H2 production in carbonate-rich, low temperature aqueous environments. Planet Space Sci 96:51–61
Nicolet Y, Piras C, Legrand P, Hatchikian CE, Fontecilla-Camps JC (1999) Desulfovibrio desulfuricans iron hydrogenase: the structure shows unusual coordination to an active site Fe binuclear center. Structure 7:13–23
Nitschke W, McGlynn SE, Milner-White EJ, Russell MJ (2013) On the antiquity of metalloenzymes and their substrates in bioenergetics. Biochim Biophys Acta (BBA)-Bioenergetics 1827:871–881
Nozaka T (2012) Petrological constraints on hydrogen production during serpentinization: a review. Japanese Magazine of Mineralogical and Petrological Sciences 41:174–184
O’Neil J, Carlson RW, Francis D, Stevenson RK (2008) Neodymium-142 evidence for hadean mafic crust. Science 321:1828–1831
Ogata H, Nishikawa K, Lubitz W (2015) Hydrogens detected by subatomic resolution protein crystallography in a [NiFe] hydrogenase. Nature 520:571–574
Ohta H, Maruyama S, Takahashi E, Watanabe Y, Kato Y (1996) Field occurrence, geochemistry and petrogenesis of the Archean mid-oceanic ridge basalts (AMORBs) of the Cleaverville area, Pilbara craton, Western Australia. Lithos 37:199–221
Ohtake M, Matsunaga T, Haruyama J, Yokota Y, Morota T, Honda C, Hirata N (2009) The global distribution of pure anorthosite on the moon. Nature 461:236–240
Pizzarello S, Cronin JR (2000) Non-racemic amino acids in the Murray and Murchison meteorites. Geochim Cosmochim Acta 64:329–338
Ragsdale SW (2004) Life with carbon monoxide. Crit Rev Biochem Mol Biol 39:165–195
Rempfert KR, Miller HM, Bompard N, Nothaft D, Matter JM, Kelemen P, Fierer N, Templeton AS (2017) Geological and geochemical controls on subsurface microbial life in the Samail ophiolite, Oman. Front Microbiol 8:56
Rhodes, J. M., Rodgers, K. V., Bansal, B. M., Wiesmann, H., Shih, C., Nyquist, L. E., Hubbard, N. J., 1974. The relationships between geology and soil chemistry at the Apollo 17 landing site. In Lunar and planetary science conference proceedings (Vol. 5, pp. 1097–1117)
Rodriguez-Garcia M, Surman AJ, Cooper GJ, Suárez-Marina I, Hosni Z, Lee MP, Cronin L (2015) Formation of oligopeptides in high yield under simple programmable conditions. Nat Commun 6:8385
Russell MJ, Hall AJ (1997) The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J Geol Soc Lond 154:377–402
Russell, M. J., Hall, A. J., 2006. The onset and early evolution of life. In: evolution of early Earth’s atmosphere hydrosphere, and biosphere-constraints from ore deposits (eds. S. E. Kesler and H. Ohmoto), Geochemical Society of America Memoir 198, 1–32
Russell MJ, Martin W (2004) The rocky roots of the acetyl coenzyme-a pathway. Trends Biochem Sci 24:358–363
Russell MJ, Barge LM, Bhartia R, Bocanegra D, Bracher PJ, Branscomb E, Kidd R, McGlynn S, Meier DH, Nitschke W, Shibuya T, Vance S, White L, Kanik I (2014) The drive to life on wet and icy worlds. Astrobiology 14:308–343
Sakata K, Yabuta H, Kondo T (2014) Effects of metal ions and pH on the formation and decomposition rates of di-and tri-peptides in aqueous solution. Geochem J 48:219–230
Santosh M, Arai T, Maruyama S (2017) Hadean earth and primordial continents: the cradle of prebiotic life. Geosci Front 8:309–327
Sato T, Yoshiya K, Maruyama S (2019) History of the hadean “living microfossil” and ultra-reducing environments. J Geogr (Chigaku Zasshi), (in Japanese with English abstract), accepted
Sleep NH (2010) The hadean-archaean environment. Cold Spring Harb Perspect Biol 2:a002527
Sossi PA, Eggins SM, Nesbitt RW, Nebel O, Hergt JM, Campbell IH, O’Neill HC, Kranendonk MV, Davies DR (2016) Petrogenesis and geochemistry of Archean komatiites. J Petrol 57:147–184
Suda K, Ueno U, Yoshizaki M, Nakamura H, Kurokawa K, Nishiyama E, Yoshino K, Hongoh Y, Kawachi K, Omori S, Yamada K, Yoshida N, Maruyama S (2014) Origin of methane in serpentinite-hosted hydrothermal systems: the CH4–H2–H2O hydrogen isotope systematics of the Hakuba Happo hot spring. Earth Planet Sci Lett 386:112–125
Suzuki S, Ishii S, Wu A, Cheung A, Tenney A, Wanger G, Kuenen JG, Nealson KH (2013) Microbial diversity in the cedars, an ultrabasic, ultrareducing, and low salinity serpentinizing ecosystem. Proc Natl Acad Sci USA 110:15336–15341
Suzuki S, Ishii S, Hoshino T, Rietze A, Tenney A, Morrill PL, Inagaki F, Kuenen JG, Nealson KH (2017) Unusual metabolic diversity of hyperalkaliphilic microbial communities associated with subterranean serpentinization at the cedars. ISME J:1–15
Svetlitchnyi V, Dobbeck H, Meyer-Klaucke W, Meins T, Thiele B, Römer P, Huber R, Meyer O (2004) A functional Ni–Ni–[4Fe4S] cluster in the monomeric acetyl-CoA synthase from Carboxydothermus hydrogenoformans. Proc Natl Acad Sci USA 101:446–451
Valley JW, Cavosie AJ, Ushikubo T, Reinhard DA, Lawrence DF, Larson DJ, Clifton PH, Kelly TF, Wilde SA, Moser DE, Spicuzza MJ (2014) Hadean age for a post-magma ocean zircon confirmed by atom-probe tomography. Nat Geosci 7:219–223
Vignais PM, Billoud B (2007) Occurrence, classification, and biological function of hydrogenases: an overview. Chem Rev 107:4206–4272
Volbeda A, Darnault C, Tan X, Lindahl PA, Fontecilla-Camps JC (2009) Novel domain arrangement in the crystal structure of a truncated acetyl-CoA synthase from Moorella thermoacetica. Biochemistry 48:7916–7926
Wänke, H., Baddenhausen, H., Dreibus, G., Jagoutz, E., Kruse, H., Palme, H., Teschke, F., 1973. Multielement analyses of Apollo 15, 16, and 17 samples and the bulk composition of the moon. In Lunar and planetary science conference proceedings (Vol. 4, p. 1461)
Weiss MC, Sousa FL, Mrnjavac N, Neukirchen S, Roettger M, Nelson-Sathi S, Martin WF (2016) The physiology and habitat of the last universal common ancestor. Nat Microbiol 1:16116
Westall F, Hickman-Lewis K, Hinman N, Gautret P, Campbell KA, Bréhéret JG, Foucher F, Hubert A, Sorieul S, Dass AV, Kee TP, Georgelin T, Brack A (2018) A hydrothermal-sedimentary context for the origin of life. Astrobiology 18:259–293
Wilde SA, Valley JW, Peck WH, Graham CM (2001) Evidence from detrital zircons for the existence of continental crust and oceans on the earth 4.4 Gyr ago. Nature 409:175–178
Zahnle K, Arndt N, Cockell C, Halliday A, Nisbet E, Selsis F, Sleep NH (2007) Emergence of a habitable planet. Space Sci Rev 129:35–78
Acknowledgments
We thank Chief Editor Alan W. Schwartz and anonymous reviewer whose faithful and constructive comments improved our manuscript. We thank Dr. Jim Cleaves for discussion and comments, Ms. Reiko Hattori for technical assistance completing this paper and Ms. Lucy Kwok for improving our English. This work was supported by JSPS grants (No. 26106001, 26106002) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yoshiya, K., Sato, T., Omori, S. et al. The Birthplace of Proto-Life: Role of Secondary Minerals in Forming Metallo-Proteins through Water-Rock Interaction of Hadean Rocks. Orig Life Evol Biosph 48, 373–393 (2018). https://doi.org/10.1007/s11084-019-09571-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-019-09571-y