Abstract
A recent study related to aquaponics has shown that hydroponic lettuce grown in aquaculture-derived supplemented water grew significantly better than lettuce grown in a conventional hydroponic system. The principal objective of this study was to verify this finding in a larger setup. Even though the aquaculture water that was added to the aquaculture-based hydroponic system contained relatively high amounts of sodium, we were still able to observe an enhanced growth performance of the lettuce in that system compared to the lettuce grown in the conventional hydroponic nutrient solution. The lettuce final fresh weight was 7.9%, and its final dry weight even 33.2% higher than the one of the hydroponic control.
Similar content being viewed by others
Introduction
Aquaponics has received broad attention in both the civil society and the scientific community. Traditionally, aquaponics is an integrated multi-trophic food production system that combines the elements of recirculating aquaculture system (RAS) and hydroponics in one-loop recirculating systems (Knaus and Palm 2017; Schmautz et al. 2016; Yogev et al. 2016). Data from several studies have also showed that aquaponic systems perform well in both water and resource usage (Reyes Lastiri et al. 2016; Suhl et al. 2016). Its lack of the ability to provide optimal conditions for both fish and plants is most likely the main reason why it is currently mainly being used for educational purposes (Villarroel et al. 2016). This is because one-loop aquaponic systems currently cannot compete with yields found in conventional hydroponic systems (Delaide et al. 2016). The commercial breakthrough is still outstanding (dos Santos 2016; Goddek et al. 2015). Recent trends in aquaponics have led to a renewed approach that aims at optimising the conditions in both system components. Kloas et al. (2015) and Goddek et al. (2016) both showed that nutrient concentrations in one-loop systems are suboptimal and suggest to focus on so-called decoupled multi-loop systems to allow optimal conditions (i.e. in nutrient concentrations, temperature, pH, etc.) for both fish and plants. This paper will primarily focus on such multi-loop aquaponic systems.
Although most of the turnover in aquaponic systems is made with the sale of plants (i.e. the sale of the fish only represents a minor proportion of the turnover), only two studies have attempted to investigate the plant growth potential in aquaponic systems on commercial hydroponic nutrient levels. Delaide et al. (2016) claimed that aquaponic-grown lettuce in significantly similar chemical nutrient solutions shows a growth advantage of approximately 40% over hydroponics. Contrary to that, another recent study (Suhl et al. 2016) that investigated tomato growth under similar conditions failed to show any significant production advantage. Taken together, there is a lack of formal experimental data with respect to growth of plants in RAS-based nutrient solutions on hydroponic levels. Consequently, this study was set out to confirm or disaffirm the findings of Delaide et al. (2016) in a larger setup using RAS water versus a typical hydroponic reference.
Materials and methods
Experimental setup
Two nutrient flow technique (NFT) systems were placed in a plastic tunnel greenhouse in Bleiswijk, The Netherlands, during a warm August–October season. The gullies of the NFT systems, each system servicing 16 gullies of 7.7 m long, were mounted in two blocks of eight rows and distributed in alternation. The system of cross-over NFT was applied, leading to a drip irrigation nozzle for every plant, while drain water was directly collected and did not affect neighbouring crops. These individual water nozzles gave a water flow of 2 L per hour. No additional CO2 was applied. The recirculation container of each NFT system contained 250 L of water.
Gullies were planted with 38 lettuces each, leading to a planting density of 12 heads per square meter. Hydrologically speaking, this approach, however, cannot be considered as a repetition. The scheme as well as a picture of the experimental setup can be seen in Figs. 1 and 2.
The hydroponic treatment tank has been filled up with rain water continuously and the RAS treatment tank with 30% RAS water and 70% rain water. To both tanks, hydroponic nutrient solutions (General Hydroponics, FloraMicro, FloraGrow and FloraBloom, 3:2:1 mixing ratio) were added daily. The RAS water was taken from a RAS system cultivating carps just hours before it was added to the hydroponic system. The climate was monitored (temperature, relative humidity (RH), irradiation). Water loss due to evapotranspiration and leakage was replaced continuously in the basin, while the electrical conductivity (EC) and acidity (pH) were measured daily, and kept constant on 1800 μS cm−2 and pH 5.0–6.0 respectively. Every 2 weeks, 40 L RAS water was added to the hydroponic sump.
Water analysis
Once every 2 weeks, water of both systems, as well as the RAS water from an experimental carp RAS system in Wageningen, has been sent to the lab for analysis of the plant-relevant ion composition. The water samples have been measured by the commercial lab Groen Agro Control, Delft, The Netherlands, using HPLC equipment according to the ISO 17025 norm.
Lettuce
Butterhead lettuce (variety Cosmopolia, RZ) were sown in 4 × 4 peat blocks and put directly into the system on the 23rd of August 2016. Seven weeks after planting (11th of October), 20 lettuce shoots were randomly selected, harvested and weighed individually. Prior to sending in the milled lettuce shoots for lead analysis, the lettuce heads of each system were cut into small pieces, weighed and merged in brown paper bags (40 × 20 × 20 cm) and dried (for 24 h at 103 °C) to determine their dry weight. The leaf analysis of the nutrients was performed with an ICP-OES by Groen Agro Control according to their certified analysis protocol.
Statistical analysis
Data are presented as mean ± standard deviation (SD) and ranges respectively of n samples. Analysis of statistical significance and ANOVA were conducted in R (R Core Team 2013). Furthermore, the nonparametric two-sample Kolmogorov–Smirnov test was used to test whether the two (i.e. in the RAS and HP system) Na concentration probability distributions differ. Genstat software was used to conduct a principal component analysis with respect to the lettuce’s nutrient composition.
Results and discussion
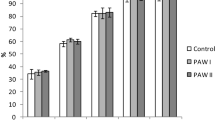
The EC was maintained constant throughout the experiment. Average day temperatures were 17.7 °C, while average outside irradiation was 1584.66 J cm−2 day−1 and light transmission index of the foil greenhouse was 57.7%. The nutrient concentrations of the RAS water and the respective systems can be seen in Tables 1 and 2. What attracts attention is that the difference in sodium concentrations is significant (Table 1, Fig. 3). This difference can be attributed to the RAS water (Table 1). In RAS systems, sodium chloride is often added to counteract stress, restore osmoregulation and prevent and control diseases. Adding sodium chloride in aquaponic systems, however, is not common practice, as it is known to inhibit plant growth.
Throughout the experiment, a visually different growth was observed. At harvest, the lettuce from the RAS water system was further developed than the lettuce that was grown in pure hydroponic water. This was indicated by the fact that the RAS-system lettuce was in the heading stage at harvest, while the hydroponic-system lettuce was still in the previous cupping stage (Fig. 4). Table 3, which shows the fresh weight of the plants, rejects the possible assumption that the faster development could have been caused by physiological stress due to high sodium chloride values. Although lettuce is known to be a relatively sodium-insensitive plant, it is remarkable to see that the fresh weight difference is highly significant to the advantage of the RAS-system lettuce. Its final fresh weight was 7.9%, and its final dry weight even 33.2% higher than the one of the hydroponic control. It is also worth mentioning that the dry matter (%) of the lettuce grown in the RAS was 2.5 times higher (7.8%) than the lettuce grown in the pure hydroponic environment (3.1%). While in our case the chemical composition of the RAS water was suboptimal for plant production, the findings are still consistent with those of Delaide et al. (2016), even though our observations in final weight differences were lower.
Despite repetitive addition of sodium-rich RAS water, the treatment did not show accumulation to the extent expected (Fig. 5). This could be due to solely increased uptake of sodium by the plants. Based on modelled evapotranspiration by the plants (i.e. Penman Monteith Equation), calculated uptake concentrations were found to be 0.04 and 0.21 mmol L−1 for the control and RAS treatment respectively. Leakage of the system, combined with continuous refill of the water reservoir with rain water, would explain the decrease of sodium concentration over time. EC levels remained the same due to daily application of nutrient solution. These concentrations are still lower than uptake concentrations of 0.5–0.8 mmol L−1 found under high sodium levels in the nutrient solution (Sonneveld and Voogt 2009). Taking a look at the lettuce leaf composition (Table 4) shows that the uptake of the other salts K, Ca, Mg, and P also was stimulated in the RAS-water growth environment (Fig. 6), while the uptake of the micronutrients Zn and Mo was rather inhibited compared with the lettuce grown in the hydroponic environment. In the same figure, it can be seen that the uptake of the macronutrients P, Mg, Ca, Na and K correlates with the RAS-system-grown lettuces, and the micronutrients Cu, B, Mo and Zn correlate with the HP-system-grown lettuces. Fe and N rather seem to be independent variables. It could be hypothesised that a possible interaction with micro-organism in the rhizosphere caused a better photosynthesis and nutrient uptake. However, we do not know what the uptake rate of the hydroponic control would be if the same amount of Na was added there. Consequently, it is yet to find out which plant species are able to take up these high amounts of sodium and to what degree different species would have to be combined in one system to avoid accumulations.
A principal component analysis of the lettuce leaf composition (Table 4)
In addition, Fig. 4 shows the harvested crops. It can clearly be seen that the leaves of the RAS-system lettuce overlap and cover the growing point of the plant, which indicated that the lettuce is being in the heading stage, while the hydroponic system lettuce is still in the cupping stage. The cupping stage is prior to the heading stage, where the inner leaves begin to curl inwards on the edges.
Conclusion
The purpose of the this study was to test the hypothesis that lettuce shows a better growth performance in hydroponic nutrient solutions based on RAS-derived water (2016). The results of this study let us presume that lettuce growth might still outperform hydroponic growth rates under chemically suboptimal (i.e. high sodium chloride) conditions. However, this study does not explain this phenomenon. A possible explanation could be the beneficial interaction between microorganisms from the RAS water and the plant roots. Earlier, Mayak et al. (2004) described conferred stress resistance in tomato against sodium due to symbiosis with bacteria. With a larger setup and uniform cultivation conditions, this study does not falsify earlier findings by Delaide et al. (2016) that integration between fish production and plant cultivation—aquaponics—could lead to stronger plant development. Further studies are currently carried out in order to validate this hypothesis. Future studies should also clarify whether similar conclusions could be drawn, if the same amount of sodium chloride as in the RAS-derived water were added to the hydroponic group, or studies where RAS-derived water was sterilised before supplying it to the root zone.
References
Delaide B, Goddek S, Gott J, Soyeurt H, Jijakli M (2016) Lettuce (Lactuca sativa L. var. Sucrine) growth performance in complemented aquaponic solution outperforms hydroponics. Water 8:467. https://doi.org/10.3390/w8100467
dos Santos MJPL (2016) Smart cities and urban areas—aquaponics as innovative urban agriculture. Urban For Urban Green 20:402–406. https://doi.org/10.1016/j.ufug.2016.10.004
Goddek S, Delaide B, Mankasingh U, Ragnarsdottir K, Jijakli H, Thorarinsdottir R (2015) Challenges of sustainable and commercial aquaponics. Sustainability 7:4199–4224. https://doi.org/10.3390/su7044199
Goddek S, Espinal CA, Delaide B, Jijakli MH, Schmautz Z, Wuertz S, Keesman KJ (2016) Navigating towards decoupled aquaponic systems: a system dynamics design approach. Water(Switzerland) 8:303. https://doi.org/10.3390/W8070303
Kloas W, Groß R, Baganz D, Graupner J, Monsees H, Schmidt U, Staaks G, Suhl J, Tschirner M, Wittstock B, Wuertz S, Zikova A, Rennert B (2015) A new concept for aquaponic systems to improve sustainability, increase productivity, and reduce environmental impacts. Aquac Environ Interact 7:179–192. https://doi.org/10.3354/aei00146
Knaus U, Palm HW (2017) Effects of fish biology on ebb and flow aquaponical cultured herbs in northern Germany (Mecklenburg Western Pomerania). Aquaculture 466:51–63. https://doi.org/10.1016/j.aquaculture.2016.09.025
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42:565–572. https://doi.org/10.1016/j.plaphy.2004.05.009
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL. http://www.R-project.org/
Reyes Lastiri D, Slinkert T, Cappon HJ, Baganz D, Staaks G, Keesman KJ (2016) Model of an aquaponic system for minimised water, energy and nitrogen requirements. Water Sci Technol wst2016127. https://doi.org/10.2166/wst.2016.127
Schmautz Z, Loeu F, Liebisch F, Graber A, Mathis A, Griessler Bulc T, Junge R (2016) Tomato productivity and quality in aquaponics: comparison of three hydroponic methods. Water 8:533. https://doi.org/10.3390/w8110533
Sonneveld C, Voogt W (2009) Plant nutrition in future greenhouse production, in: plant nutrition of greenhouse crops. Springer Netherlands, pp 393–403. https://doi.org/10.1007/978-90-481-2532-6_17
Suhl J, Dannehl D, Kloas W, Baganz D, Jobs S, Scheibe G, Schmidt U (2016) Advanced aquaponics: evaluation of intensive tomato production in aquaponics vs. conventional hydroponics. Agric Water Manag 178:335–344. https://doi.org/10.1016/j.agwat.2016.10.013
Villarroel M, Junge R, Komives T, König B, Plaza I, Bittsánszky A, Joly A (2016) Survey of aquaponics in Europe. Water 8:468. https://doi.org/10.3390/w8100468
Yogev U, Barnes A, Gross A (2016) Nutrients and energy balance analysis for a conceptual model of a three loops off grid, aquaponics. Water 8:589. https://doi.org/10.3390/W8120589
Acknowledgements
Furthermore, we would like to thank Paul Keizer (Biometris, WUR) for the statistical supervision and Karel Keesman for critically reviewing this paper.
Funding
This research was partially supported by Aquaponik Manufaktur GmbH and the Dutch ministry of Economic Affairs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Lettuce growth performance was enhanced in the RAS-based hydroponic system.
• Exposing the lettuce to significantly higher sodium levels did not lead to growth retardation.
• Sodium accumulation did not occur in the RAS-based hydroponic system.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Goddek, S., Vermeulen, T. Comparison of Lactuca sativa growth performance in conventional and RAS-based hydroponic systems. Aquacult Int 26, 1377–1386 (2018). https://doi.org/10.1007/s10499-018-0293-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-018-0293-8