Abstract
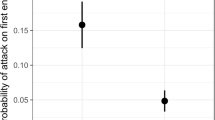
Arboreal ants are both highly diverse and ecologically dominant in the tropics. This ecologically important group is particularly useful in ongoing efforts to understand processes that regulate species diversity and coexistence. Our study addresses how polydomy can influence patterns of nest occupation in competing arboreal ants. We examined the spatial structure of nest occupation (nest distance, abundance and density) in three polydomous co-occurring twig-nesting ant species (Pseudomyrmex simplex, P. ejectus and P. PSW-53) by mapping twigs occupied by ants from each species within plots in our study site. We then used two colony structure estimators (intraspecific aggression and cuticular hydrocarbon variation) to determine the relative degree of polydomy for each species. All work was conducted in coffee agroforests in Chiapas, Mexico. Our results revealed that the two species with highest abundance and nest density were also highly polydomous, where both species had either single or multiple non-aggressive colonies occupying nests on a large spatial scale (greater than the hectare level). Our results also indicate that the species with the lowest abundance and density is less polydomous, occupying several overlapping and territorial colonies at the hectare level in which multiple colonies never co-occur on the same host plant. These results contribute evidence that successful coexistence and highly polydomous colony structure may allow ants, through reduced intraspecific aggression, to successfully occupy more nests more densely than ant species that have multiple territorial colonies. Furthermore our study highlights the importance of considering intraspecific interactions when examining community assembly of ants.





Similar content being viewed by others
References
AntWeb (2015) Available at http://www.antweb.org/ (accessed 10 October 2015)
Bluthgen N, Stork NE (2007) Ant mosaics in a tropical rainforest in Australia and elsewhere: a critical review. Austral Ecol 32:93–104
Bluthgen N, Verhaagh M, Goitia W et al (2000) How plants shape the ant community in the Amazonian rainforest canopy: the key role of extrafloral nectaries and homopteran honeydew. Oecologia 125:229–240. doi:10.1007/s004420000449
Blüthgen N, Stork NE, Fiedler K (2004) Bottom-up control and co-occurrence in complex communities: honeydew and nectar determine a rainforest ant mosaic. Oikos 106:344–358
Buczkowski G (2011) Colony spatial structure in polydomous ants: complimentary approaches reveal different patterns. Insectes Soc 59:241–250. doi:10.1007/s00040-011-0211-9
Crozier RH, Dix MW (1979) Analysis of two genetic models for the innate components of colony odor in social Hymenoptera. Behav Ecol Sociobiol 4:217–224. doi:10.1007/BF00297645
Davidson D (1997) The role of resource imbalances in the evolutionary ecology of tropical arboreal ants. Biol J Linn Soc 61:153–181. doi:10.1006/bijl.1996.0128
Davidson DW, Cook SC et al (2003) Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300:969–973. doi:10.1126/science.1082074
De Vega C, Herrera CM, Dötterl S (2014) Floral volatiles play a key role in specialized ant pollination. Perspect Plant Ecol Evol Syst 16:32–42. doi:10.1016/j.ppees.2013.11.002
Debout G, Schatz B, Elias M, Mckey D (2007) Polydomy in ants: what we know, what we think we know, and what remains to be done. J Linn Soc 90:319–348
Dejean A, Corbara B, Orivel J, Leponce M (2007) Rainforest canopy ants: the implications of territoriality and predatory behavior. Funct Ecosyst Communities 1:105–120
Dejean A, Djiéto-Lordon C, Céréghino R, Leponce M (2008) Ontogenetic succession and the ant mosaic: an empirical approach using pioneer trees. Basic Appl Ecol 9:316–323. doi:10.1016/j.baae.2007.03.001
Dejean A, Ryder S, Bolton B et al (2015) How territoriality and host-tree taxa determine the structure of ant mosaics. Sci Nat. doi:10.1007/s00114-015-1282-7
Feldhaar H, Fiala B, Gadau J (2005) A shift in colony founding behaviour in the obligate plant-ant Crematogaster (Decacrema) morphospecies 2. Insectes Soc 52:222–230. doi:10.1007/s00040-004-0797-2
Foitzik S, Sturm H, Pusch K et al (2007) Nestmate recognition and intraspecific chemical and genetic variation in Temnothorax ants. Anim Behav 73:999–1007. doi:10.1016/j.anbehav.2006.07.017
Heller NE, Ingram KK, Gordon DM (2008) Nest connectivity and colony structure in unicolonial Argentine ants. Insectes Soc 55:397–403. doi:10.1007/s00040-008-1019-0
Helms KR, Helms Cahan S (2012) Large-scale regional variation in cooperation and conflict among queens of the desert ant Messor pergandei. Anim Behav 84:499–507. doi:10.1016/j.anbehav.2012.05.019
Herbers JM, DeHeer CJ, Foitzik S (2001) Conflict over sex allocation drives conflict over reproductive allocation in perennial social insect colonies. Am Nat 158:178–192. doi:10.1086/321312
Hölldobler B, Wilson EO (1990) The Ants. Harvard University, Cambridge
Holway D (1998) Loss of Intraspecific Aggression in the Success of a Widespread Invasive Social Insect. Science 282:949–952. doi:10.1126/science.282.5390.949 (80-)
Holway D, Lach L, Suarez AV et al (2002) The causes and consequences of ant invasions. Annu Rev Ecol Syst 33:181–233. doi:10.1146/annurev.ecolsys.33.010802.150444
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393. doi:10.1146/annurev.ento.50.071803.130359
Izzo TJ, Bruna EM, Vasconcelos HL, Inouye BD (2009) Cooperative colony founding alters the outcome of interspecific competition between Amazonian plant-ants. Insectes Soc 56:341–345. doi:10.1007/s00040-009-0029-x
Jackson D (1984) Ant distribution patterns in a Cameroonian cocoa plantation: investigation of the ant mosaic hypothesis. Oecologia 62:318–324. doi:10.1007/BF00384263
Jiménez-Soto E, Philpott SM (2015) Size matters: nest colonization patterns for twig-nesting ants. Ecol Evol 5:3288–3298. doi:10.1002/ece3.1555
Katritzky AR, Chen K, Maran U, Carlson D (2000) QSPR correlation and predictions of GC retention indexes for methyl-branched hydrocarbons produced by insects. Anal Chem 72:101–109. doi:10.1021/ac990800w
Kautz S, Ballhorn DJ, Kroiss J et al (2012) Host plant use by competing acacia-ants: mutualists monopolize while parasites share hosts. PLoS One 7:1–10. doi:10.1371/journal.pone.0037691
Klein R (1987) Colony structures of three species of Pseudomyrmex (Hymenoptera: Formicidae: Pseudomyrmecinae) in Florida. In: Chemistry and biology of social insects. Velag J. Peperny, Munich, p 107–108
Krasnec MO, Breed MD (2013) Colony-specific cuticular hydrocarbon profile in Formica argentea ants. J Chem Ecol 39:59–66. doi:10.1007/s10886-012-0227-2
Lenoir A, Fresneau D, Errard C, Hefetz A (1999) Individuality and colonial identity in ants: the emergence of the social representation concept. In: Detrain C, Deneubourg J, Pasteels J (eds) Information Processing in Social Insects. Birkhauser Verlag Ag, Basel, pp 219–237
Lenoir A, Hefetz A, Simon T, Soroker V (2001) Comparative dynamics of gestalt odour formation in two ant species Camponotus fellah and Aphaenogaster senilis (Hymenoptera: Formicidae). Physiol Entomol 26:275–283
Liere H, Larsen A (2010) Cascading trait-mediation: disruption of a trait-mediated mutualism by parasite-induced behavioral modification. Oikos 119:1394–1400. doi:10.1111/j.1600-0706.2010.17985.x
Livingston GF, Jackson D (2014) Spatial clustering of twig nesting ants corresponds with metacommunity assembly processes. Ecología austral 24:343–349
Livingston GF, Philpott SM (2010) A metacommmunity approach to co-occurrence patterns and the core-satellite hypothesis in a community of tropical arboreal ants. Ecol Res 25:1129–1140. doi:10.1007/s11284-010-0738-7
Majer JD (1972) The ant mosaic in Ghana cocoa farms. Bull Entomol Res 62:151. doi:10.1017/S0007485300047593
Majer JD, Delabie JHC, Smith MRB (1994) Arboreal ant community patterns in Brazilian cocoa farms. Biotropica 26:73–83. doi:10.2307/2389112
Mathews CR, Bottrell DG, Brown MW (2011) Interactions between extrafloral nectaries, ants (Hymenoptera: Formicidae), and other natural enemies affect biological control of Grapholita molesta (Lepidoptera: Tortricidae) on peach (Rosales: Rosaceae). Environ Entomol 40:42–51. doi:10.1603/EN10161
Overson R, Gadau J, Clark RM et al (2014) Behavioral transitions with the evolution of cooperative nest founding by harvester ant queens. Behav Ecol Sociobiol 68:21–30. doi:10.1007/s00265-013-1618-2
Philpott SM, Armbrecht I (2006) Biodiversity in tropical agroforests and the ecological role of ants and ant diversity in predatory function. Ecol Entomol 31:369–377
Philpott Stacy M, Foster PF (2005) Nest-site limitation in coffee agroecosystems: artificial nests maintain diversity of arboreal ants. Ecol Appl 15:1478–1485
Powell S, Costa AN, Lopes CT, Vasconcelos HL (2011) Canopy connectivity and the availability of diverse nesting resources affect species coexistence in arboreal ants. J Anim Ecol 80:352–360. doi:10.1111/j.1365-2656.2010.01779.x
R Development Core Team (2013) A language and environment for statistical computing. R Development Core Team, Vienna, Austria. Available at http://www.R-project.org
Ribas CR, Schoereder JH (2002) Are all ant mosaics caused by competition? Oecologia 131:606–611. doi: 10.1007/s00442-002-0912-x
Room PM (1971) The relative distributions of ant species in Ghana’s Cocoa farms. J Anim Ecol 40:735–751
Sanders NJ, Gotelli NJ, Heller NE, Gordon DM (2003) Community disassembly by an invasive species. Proc Natl Acad Sci USA 100:2474–2477. doi:10.1073/pnas.0437913100
Sanders NJ, Crutsinger GM, Dunn RR et al (2007) An ant mosaic revisited: dominant ant species disassemble arboreal ant communities but co-occur randomly. Biotropica 39:422–427. doi:10.1111/j.1744-7429.2007.00263.x
Suarez A, Tsutsui N, Holway D, Case T (1999) Behavioral and genetic differentiation between native and introduced populations of the Argentine ant. Biol Invasions 1:43–53. doi:10.1023/A:1010038413690
Suarez AV, Holway D, Liang D et al (2002) Spatiotemporal patterns of intraspecific aggression in the invasive Argentine ant. Anim Behav 64:697–708. doi:10.1006/anbe.2002.4011
Taylor B (1977) The ant mosaic on cocoa and other tree crops in Western Nigeria. Ecol Entomol 2:245–255. doi:10.1111/j.1365-2311.1977.tb00887.x
Torres CW, Brandt M, Tsutsui ND (2007) The role of cuticular hydrocarbons as chemical cues for nestmate recognition in the invasive Argentine ant (Linepithema humile). Insectes Soc 54:363–373. doi:10.1007/s00040-007-0954-5
Tsutsui ND (2004) Scents of self: the expression component of self/non- self recognition systems. Ann Zool Fennici 41:713–727
Tsutsui ND, Suarez AV, Grosberg RK (2003) Genetic diversity, asymmetrical aggression, and recognition in a widespread invasive species. Proc Natl Acad Sci USA 100:1078–1083. doi:10.1073/pnas.0234412100
Vander Meer R, Morel L (1998) Nestmate Recognition in Ants. In: Vander Meer RK, Breed MD, Espelie KE, Winston ML (eds) Pheromone communication in social insects: ants, wasps, bees and termites. Westview Press, Boulder, CO, p 79–103
Vandermeer J, Perfecto I, Philpott SM (2008) Clusters of ant colonies and robust criticality in a tropical agroecosystem. Nature 451:457–459. doi:10.1038/nature06477
Vandermeer J, Perfecto I, Philpott S (2010) Ecological complexity and pest control in organic coffee production: uncovering an autonomous ecosystem service. Bioscience 60:527–537. doi:10.1525/bio.2010.60.7.8
Vasconcelos HL (1993) Ant colonization of Maieta guianensis seedlings, an Amazon ant-plant. Oecologia 95:439–443. doi:10.1007/S00442-004-V
Ward PS (1985) The Nearctic species of the genus Pseudomyrmex (Hymenoptera: Formicidae). Quaest Entomol 21:209–246
Yitbarek S, Philpott SM. Dominance hierarchies drive local twig-nesting ant abundance patterns in a tropical agroecosystem (Unpublished data)
Acknowledgments
G. Lopez Bautista, G. Domíngez-Martínez, and F. Sánchez-López assisted with field collection and sample processing. We thank Finca Irlanda for allowing us to conduct research on the farm. We thank SEMARNAT (Secretaria de Medio Ambiente y Recursos Naturales) for permission to collect and export samples and J. Rojas and E. Chamé Vasquez for facilitating the process of acquiring permits. N. Tsutsui provided helpful comments on the manuscript. Funding was provided by a Packard Foundation Grant to SRR, National Science Foundation DEB-1262086 to SMP, and National Science Foundation GRFP DGE 1106400 and National Institutes of Health Award Number K12GM000708 to KAM.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mathis, K.A., Philpott, S.M. & Ramirez, S.R. Variation in spatial scale of competing polydomous twig-nesting ants in coffee agroecosystems. Insect. Soc. 63, 447–456 (2016). https://doi.org/10.1007/s00040-016-0489-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-016-0489-8