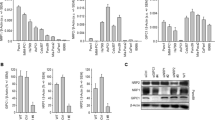
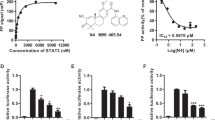
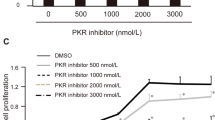
Abstract
Pancreatic carcinoma is one of the most aggressive tumors, and, being refractory to conventional therapies, is an excellent target for new therapeutic approaches. Based on our previous finding of high HMGA1 expression in pancreatic cancer cells compared to normal pancreatic tissue, we evaluated whether suppression of HMGA1 protein expression could be a treatment option for patients affected by pancreatic cancer. Here we report that HMGA1 proteins are overexpressed in pancreatic carcinoma cell lines, and their downregulation through an adenovirus carrying the HMGA1 gene in an antisense orientation (Ad Yas-GFP) results in the death of three human pancreatic carcinoma cell lines (PANC1, Hs766T and PSN1). Pretreatment of PANC1 and PSN1 cells with Ad Yas-GFP suppressed and reduced, respectively, their ability to form xenograft tumors in nude mice. To further verify the role of HMGA1 in pancreatic tumorigenesis, we used a HMGA1 antisense phosphorothioate oligodeoxynucleotide (ODN); its addition induced a decrease in HMGA1 protein levels and a significant reduction of the proliferation rate of PANC1-, Hs766T- and PSN1-treated cells. Therefore, suppression of HMGA1 protein synthesis by an HMGA1 antisense approach seems to be a feasible treatment strategy in pancreatic carcinomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Warshaw AL, Fernandez-del Castillo C . Pancreatic carcinoma. N Engl J Med. 1992;326:455–465.
Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–731 discussion 731–733.
Landis SH, Murray T, Bolden S, Wingo PA . Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31.
Johnson KR, Lehn DA, Elton TS, Barr PJ, Reeves R . Complete murine cDNA sequence, genomic structure, and tissue expression of the high mobility group protein HMG-I(Y). J Biol Chem. 1988;263:18338–18342.
Johnson KR, Lehn DA, Reeves R . Alternative processing of mRNAs encoding mammalian chromosomal high-mobility-group proteins HMG-I and HMG-Y. Mol Cell Biol. 1989;9:2114–2123.
Manfioletti G, Giancotti V, Bandiera A, et al. cDNA cloning of the HMGI-C phosphoprotein, a nuclear protein associated with neoplastic and undifferentiated phenotypes. Nucleic Acids Res. 1991;19:6793–6797.
Grosschedl R, Giese K, Pagel J . HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994;10:94–100.
Lovell-Badge R . Developmental genetics. Living with bad architecture. Nature. 1995;376:725–726.
Bustin M, Reeves R . High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog Nucleic Acid Res Mol Biol. 1996;54:35–100.
Zhou X, Benson KF, Ashar HR, Chada K . Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor HMGI-C. Nature. 1995;376:771–774.
Chiappetta G, Avantaggiato V, Visconti R, et al. High level expression of the HMGI (Y) gene during embryonic development. Oncogene. 1996;13:2439–2446.
Chiappetta G, Bandiera A, Berlingieri MT, et al. The expression of the high mobility group HMGI (Y) proteins correlates with the malignant phenotype of human thyroid neoplasias. Oncogene. 1995;10:1307–1314.
Tamimi Y, van der Poel HG, Karthaus HF, Debruyne FM, Schalken JA . A retrospective study of high mobility group protein I(Y) as progression marker for prostate cancer determined by in situ hybridization. Br J Cancer. 1996;74:573–578.
Fedele M, Bandiera A, Chiappetta G, et al. Human colorectal carcinomas express high levels of high mobility group HMGI(Y) proteins. Cancer Res. 1996;56:1896–1901.
Chiappetta G, Tallini G, De Biasio MC, et al. Detection of high mobility group I HMGI(Y) protein in the diagnosis of thyroid tumors: HMGI(Y) expression represents a potential diagnostic indicator of carcinoma. Cancer Res. 1998;58:4193–4198.
Bandiera A, Bonifacio D, Manfioletti G, et al. Expression of HMGI(Y) proteins in squamous intraepithelial and invasive lesions of the uterine cervix. Cancer Res. 1998;58:426–431.
Abe N, Watanabe T, Sugiyama M, et al. Determination of high mobility group I(Y) expression level in colorectal neoplasias: a potential diagnostic marker. Cancer Res. 1999;59:1169–1174.
Abe N, Watanabe T, Masaki T, et al. Pancreatic duct cell carcinomas express high levels of high mobility group I(Y) proteins. Cancer Res. 2000;60:3117–3122.
Chiappetta G, Manfioletti G, Pentimalli F, et al. High mobility group HMGI(Y) protein expression in human colorectal hyperplastic and neoplastic diseases. Int J Cancer. 2001;91:147–151.
Berlingieri MT, Manfioletti G, Santoro M, et al. Inhibition of HMGI-C protein synthesis suppresses retrovirally induced neoplastic transformation of rat thyroid cells. Mol Cell Biol. 1995;15:1545–1553.
Berlingieri MT, Pierantoni GM, Giancotti V, Santoro M, Fusco A . Thyroid cell transformation requires the expression of the HMGA1 proteins. Oncogene. 2002;21:2971–2980.
Wood LJ, Maher JF, Bunton TE, Resar LM . The oncogenic properties of the HMG-I gene family. Cancer Res. 2000;60:4256–4261.
Baldassarre G, Fedele M, Battista S, et al. Onset of natural killer cell lymphomas in transgenic mice carrying a truncated HMGI-C gene by the chronic stimulation of the IL-2 and IL-15 pathway. Proc Natl Acad Sci USA. 2001;98:7970–7975.
Fedele M, Battista S, Kenyon L, et al. Overexpression of the HMGA2 gene in transgenic mice leads to the onset of pituitary adenomas. Oncogene. 2002;21:3190–3198.
Scala S, Portella G, Fedele M, Chiappetta G, Fusco A . Adenovirus-mediated suppression of HMGI(Y) protein synthesis as potential therapy of human malignant neoplasias. Proc Natl Acad Sci USA. 2000;97:4256–4261.
Milner AE, Levens JM, Gregory CD . Flow cytometric methods of analyzing apoptotic cells. Methods Mol Biol. 1998;80:347–354.
Pellegata NS, Sessa F, Renault B, et al. K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res. 1994;54:1556–1560.
Redston MS, Caldas C, Seymour AB, et al. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res. 1994;54:3025–3033.
Rosenberg L . Pancreatic cancer: a review of emerging therapies. Drugs. 2000;59:1071–1089.
Schwarte-Waldhoff I, Volpert OV, Bouck NP, et al. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci USA. 2000;97:9624–9629.
Jaffee EM, Hruban RH, Canto M, Kern SE . Focus on pancreas cancer. Cancer Cell. 2002;2:25–28.
Hao D, Rowinsky EK . Inhibiting signal transduction: recent advances in the development of receptor tyrosine kinase and Ras inhibitors. Cancer Invest. 2002;20:387–404.
Bouvet M, Bold RJ, Lee J, et al. Adenovirus-mediated wild-type p53 tumor suppressor gene therapy induces apoptosis and suppresses growth of human pancreatic cancer. Ann Surg Oncol. 1998;5:681–688.
Ghaneh P, Greenhalf W, Humphreys M, et al. Adenovirus-mediated transfer of p53 and p16(INK4a) results in pancreatic cancer regression in vitro and in vivo. Gene Therapy. 2001;8:199–208.
Joshi US, Dergham ST, Chen YQ, et al. Inhibition of pancreatic tumor cell growth in culture by p21WAF1 recombinant adenovirus. Pancreas. 1998;16:107–113.
Dumon KR, Ishii H, Vecchione A, et al. Fragile histidine triad expression delays tumor development and induces apoptosis in human pancreatic cancer. Cancer Res. 2001;15:4827–4836.
Takeuchi M, Shichinohe T, Senmaru N, et al. The dominant negative H-ras mutant, N116Y, suppresses growth of metastatic human pancreatic cancer cells in the liver of nude mice. Gene Therapy. 2000;7:518–526.
Bardeesy N, DePinho RA . Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909.
Laheru D, Biedrzycki B, Jaffee EM . Immunologic approaches to the management of pancreatic cancer. Cancer J. 2001;7:324–337.
Mulvihill S, Warren R, Venook A, et al. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Therapy. 2001;8:308–315.
Rodicker F, Stiewe T, Zimmermann S, Putzer BM . Therapeutic efficacy of E2F1 in pancreatic cancer correlates with TP73 induction. Cancer Res. 2001;61:7052–7055.
Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD . How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264.
Elbashir SM, Harborth J, Lendeckel W, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in mammalian cell culture. Nature. 2001;411:494–498.
Caplen NJ, Parrish S, Imani F, Fire A, Morgan RA . Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci USA. 2001;98:9742–9747.
Bitko V, Barik S . Phenotypic silencing of cytoplasmic genes using sequence-specific double-stranded short interfering RNA and its application in the reverse genetics of wild type negative-strand RNA viruses. BMC Microbiol. 2001;1:34.
Acknowledgements
This study was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC), by the Programma Italia-USA sulla Terapia dei Tumori coordinated by Professor Cesare Peschle, Progetto Finalizzato “Biotecnologie” of Consiglio Nazionale delle Ricerche, Progetto “5%” of the Consiglio Nazionale delle Ricerche, and the MURST Project “Terapie antineoplastiche innovative”. We are indebted to Jean Gilder for editing the text and we are also grateful to David Fineman for his friendly support to our group.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trapasso, F., Sarti, M., Cesari, R. et al. Therapy of human pancreatic carcinoma based on suppression of HMGA1 protein synthesis in preclinical models. Cancer Gene Ther 11, 633–641 (2004). https://doi.org/10.1038/sj.cgt.7700745
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700745
Keywords
This article is cited by
-
Critical role of HMGA proteins in cancer cell chemoresistance
Journal of Molecular Medicine (2017)
-
Analysis of Ki67, HMGA1, MDM2, and RB expression in nonfunctioning pituitary adenomas
Journal of Neuro-Oncology (2017)
-
HMGA1 correlates with advanced tumor grade and decreased survival in pancreatic ductal adenocarcinoma
Modern Pathology (2010)
-
Pancreatic cancer: molecular pathogenesis and new therapeutic targets
Nature Reviews Gastroenterology & Hepatology (2009)
-
Apoptotic pathways in pancreatic ductal adenocarcinoma
Molecular Cancer (2008)