Abstract
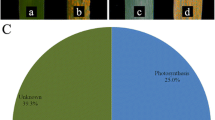
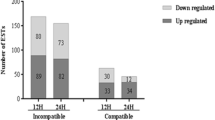
Soybean rust caused by Phakopsora pachyrhizi Sydow is a devastating foliar disease that has spread to most soybean growing regions throughout the world, including the USA. Four independent rust resistance genes, Rpp1–Rpp4, have been identified in soybean that recognize specific isolates of P. pachyrhizi. A suppressive subtraction hybridization (SSH) complementary DNA (cDNA) library was constructed from the soybean accession PI200492, which contains Rpp1, after inoculation with two different isolates of P. pachyrhizi that result in susceptible or immune reactions. Both forward and reverse SSH were performed using cDNA from messenger RNA pooled from 1, 6, 12, 24, and 48 h post-inoculation. A total of 1,728 SSH clones were sequenced and compared to sequences in GenBank for similarity. Microarray analyses were conducted on a custom 7883 soybean-cDNA clone array encompassing all of the soybean-rust SSH clones and expressed sequence tags from four other soybean cDNA libraries. Results of the microarray revealed 558 cDNA clones differentially expressed in the immune reaction. The majority of the upregulated cDNA clones fell into the functional category of defense. In particular, cDNA clones with similarity to peroxidases and lipoxygenases were prevalent. Downregulated cDNA clones included those with similarity to cell-wall-associated protein, such as extensins, proline-rich proteins, and xyloglucan endotransglycosylases.




Similar content being viewed by others
References
Alkharouf N, Khan R, Matthews B (2004) Analysis of expressed sequence tags from roots of resistant soybean infected by the soybean cyst nematode. Genome Biol 47:380–388
Alkharouf N, Jamison DC, Matthews BF (2005) Online analytical processing (OLAP): a fast and effective data mining tool for gene expression databases. J Biomed Biotechnol 2:181–188
Alkharouf N, Klink V, Chouikha I, Beard H, Macdonald M, Meyer S, Knap H, Khan R, Matthews B (2006) Timecourse microarray analyses reveal global changes in gene expression of susceptible Glycine max (soybean) roots during infection by Heterodera glycines (soybean cyst nematode). Planta 224:838–852
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G (2000) Gene Ontology: tool for the unification of biology. Nat Genet 25:25–29
Ayliffe MA, Roberts JK, Mitchell HJ, Zhang R, Lawrence GJ, Ellis JG, Pryor TJ (2002) A plant gene up-regulated at rust infection sites. Plant Physiol 129:169–180
Bateman A, Coin L, Durbin R, Finn R, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer E, Studholme D, Yeats C, Eddy S (2004) The Pfam protein families database. Nucleic Acids Res 32:D138–D141
Bonde MR, Nester SE, Austin CN, Stone CL, Frederick RD, Hartman GL, Miles MR (2006) Evaluation of virulence of Phakopsora pachyrhizi and P. meibomiae isolates. Plant Dis 90:708–716
Bradley DJ, Kjellbom P, Lamb CJ (1992) Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70:21–30
Brisson LF, Tenhaken R, Lamb C (1994) Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 6:1703–1712
Bromfield KR (1984) Soybean rust. Monograph No. 11. America Phytopathological Society, St. Paul
Bromfield KR, Hartwig EE (1980) Resistance to soybean rust [Phakopsora pachyrhizi] and mode of inheritance. Crop Sci 20:254–255
Camera SL, Gouzerh G, Dhondt S, Hoffmann L, Fritig B, Legrand M, Heitz T (2004) Metabolic reprogramming in plant innate immunity: the contributions of phenylpropanoid and oxylipin pathways. Immunol Rev 198:267–284
Cherry E, Peet C (1966) An efficient device for the rapid collection of fungal spores from infected plants. Phytopathology 56:1102–1103
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Codd E, Codd S, Salley C (1993) Providing OLAP (on-line analytical processing) to user-analysts: an IT mandate. Technical Report, EF Codd & Associates
Dardick C (2007) Comparative expression profiling of Nicotiana benthamiana leaves systemically infected with three fruit tree viruses. Mol Plant Microb Interact 20:1004–1017
Diatchenko L, Lau Y-FC, Campbell AP, Chenchik A, Moqadam F, Huang B, Lukyanov S, Gurskaya N, Sverdlov E, Siebert PD (1996) Suppression subtractive hybridization: A method for generating differentially regulated or tissue-specific cDNA probes and libraries. Proc Natl Acad Sci U S A 93:6025–6030
D’ovidio R, Roberti S, Di Giovanni M, Capodicasa C, Melaragni M, Sella L, Tosi P, Favaron F (2006) The characterization of the soybean polygalacturonase-inhibiting proteins (Pgip) gene family reveals that a single member is responsible for the activity detected in soybean tissues. Planta 224:663–645
Fehr WR, Caviness CE (1977) Stages of soybean development. Iowa State University, Ames, IA
Fei F, Tan Y, Zhou M (1996) Histopathological study of rust (Phakopsora pachyrhizi Syd.) resistance in soybeans. Oil Crops China 18:48–50
Foyer CH, Noctor G (2003) Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plantarum 119:355–364
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Golkari S, Gilbert J, Prashar S, Procunier JD (2007) Microarray analysis of Fusarium graminearum-induced wheat genes: identification of organ-specific and differentially expressed genes. Plant Biotechnol J 5:38–49
Grandbastien MA, Berry-Lowe S, Shirley BW, Meagher RB (1986) Two soybean ribulose-1,5-bisphosphate carboxylase small subunit genes share extensive homology even in distant flanking sequences. Plant Mol Biol 7:451–465
Hartman GL (1996) Highlights of soybean rust research at the Asian Vegetable Research and Development Center. In: Sinclair JB, Hartman GL (eds) Proceedings of the soybean rust workshop, 9–11 August 1995. College of Agricultural, Consumer, and Environmental Sciences, National Soybean Research Laboratory, Urbana, IL, pp 19–28
Hartwig E (1986) Identification of a fourth major gene conferring resistance to soybean rust. Crop Sci 26:1135–1136
Hartwig E, Bromfield K (1983) Relationships among three genes conferring resistance to soybean rust. Crop Sci 23:237–239
Hegde P, Qi R, Abernathy K, Gay C, Dharap S, Gaspard R, Hughes JE, Snesrud E, Lee N, Quackenbush J (2000) A concise guide to cDNA microarray analysis. Biotechniques 29:548–556
Hennings P (1903) Some new Japanese Uredinales. Hedwiga 4(suppl.):107–108
Hyten DL, Hartman GL, Nelson RL, Frederick RD, Concibido VC, Narvel JM, Cregan PB (2007) Map location of the Rpp1 locus that confers resistance to soybean rust in soybean. Crop Sci 47:835–838
Hu HY, Zhuang JY, Chai RY, Wu JL, Fan YY, Zheng KL (2006) Isolation and characterization of defense response genes involved in neck blast resistance of rice. Yi Chuan Xue Bao 33:251–261
Khan R, Alkharouf N, Beard HS, MacDonald M, Chouikha I, Meyer S, Grefenstette J, Knap H, Matthews BF (2004) Resistance mechanisms in soybean: gene expression profile at an early stage of soybean cyst nematode invasion. J Nematol 36:241–248
Kawano T (2003) Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep 21:829–837
Keogh R, Deverall B (1980) Comparison of histological and physiological responses to Phakopsora pachyrhizi in resistant and susceptible soybean. Trans Brit Mycol Soc 74:329–333
Kurkcuoglu S, Degenhardt J, Lensing J, Al-Masri AN, Gau AE (2007) Identification of differentially expressed genes in Malus domestica after application of the non-pathogenic bacterium Pseudomonas fluorescens Bk3 to the phyllosphere. J Exp Bot 58:733–741
Levine A, Tenhaken R, Dixon R, Lamb CJ (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–589
Li GS, Asiegbu FO (2004) Use of Scots pine seedling roots as an experimental model to investigate gene expression during interaction with the conifer pathogen Heterobasidion annosum. J Plant Res 117:155–162
Lin H, Doddapaneni H, Takahashi Y, Walker MA (2007) Comparative analysis of ESTs involved in grape responses to Xylella fastidiosa infection. BMC Plant Biol 7:8
Marchler-Bauer A, Anderson JB, Cherukuri PF, DeWeese-Scott C, Geer LY, Gwadz M, He S, Hurwitz DI, Jackson JD, Ke Z, Lanczycki C, Liebert CA, Liu C, Lu F, Marchler GH, Mullokandov M, Shoemaker BA, Simonyan V, Song JS, Thiessen PA, Yamashita RA, Yin JJ, Zhang D, Bryant SH (2005) CDD: a Conserved Domain Database for protein classification. Nucleic Acids Res 33:D192–D196
McLean R, Byth D (1976) Resistance of soybean to rust in Australia. Aust Plant Pathol Soc Newsl 5:34–36
McLean R, Byth D (1980) Inheritance of resistance to rust (Phakopsora pachyrhizi) in soybeans. Aust J Agric Res 31:951–956
McLean R, Byth D (1981) Histological studies of the pre-penetration development and penetration of soybeans by rust. Phakopsora pachyrhizi Syd. Aust J Agric Res 32:435–443
Melching JS, Bromfield KR, Kingsolver CH (1983) The plant pathogen containment facility at Frederick, Maryland. Plant Dis 67:717–722
Miles MR, Hartman GL, Frederick RD (2003a) Soybean rust: Is the U.S. soybean crop at risk? Online. APSnet Feature, American Phytopathological Society, St. Paul, MN
Miles MR, Hartman GL, Levy C, Morel W (2003b) Current status of soybean rust control by fungicides. Pestic Outlook 14:197–200
Miles MR, Frederick, RD, Hartman, GL (2006) Evaluation of soybean germplasm for resistance to Phakopsora pachyrhizi. Online. Plant Health Progress. DOI 10.1094/PHP-2006-0104-01-RS
Mo J, Zhu G, Chen Y, Huang L, Sun H (1994) Preliminary survey and identification of soybean varietal resistance to rust. In: Dept. of Science and Technology, MARPC; Asian Regional Center, AVRDC; and Oil Crops Research Institute, CAAS (eds) Advance of soybean rust research. Proceedings of a meeting held in 1992. Hubei Science and Technology Publishing House, Hubei, People’s Republic of China, pp 116–122
Monteros MJ, Missaoui AM, Phillips DV, Walker DR, Boerma HR (2007) Mapping and confirmation of the “Hyuuga” red-brown lesion resistance gene for Asian soybean rust. Crop Sci 47:829–836
Nelson RT, Shoemaker R (2006) Identification and analysis of gene families from the duplicated genome of soybean using EST sequences. BMC Genomic 7:204
Okushima Y, Koizumi N, Kusano T, Sano H (2000) Secreted proteins of tobacco cultured BY2 cells: identification of a new member of pathogenesis-related proteins. Plant Mol Biol 42:479–488
Panthee DR, Yuan JS, Wright DL, Marois JJ, Mailhot D, Stewart CN Jr (2007) Gene expression analysis in soybean in response to the causal agent of Asian soybean rust (Phakopsora pachyrhizi Sydow) in an early growth stage. Funct Integr Genom 7:291–301(11)
Pfaffl MW (2001) A mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24:255–265
Patil PV, Basavaraja GT (1997) A prospective source of resistance to soybean rust. Karnataka J Agric Sci 10:1241–1243
Pinto MCD, Paradiso A, Leonetti P, Gara LD (2006) Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defense and cell death. Plant J 48:784–795
Ray S, Anderson JM, Urmeev FI, Goodwin SB (2003) Rapid induction of a protein disulfide isomerase and defense-related genes in wheat in response to the hemibiotrophic fungal pathogen Mycosphaerella graminicola. Plant Mol Biol 53:741–754
Rhee SY, Beavis W, Berardini TZ, Chen G, Dixon D, Doyle A, Garcia-Hernandez M, Huala E, Lander G, Montoya M, Miller N, Mueller LA, Mundodi S, Reiser L, Tacklind J, Weems DC, Wu Y, Xu I, Yoo D, Yoon J, Zhang P (2003) The Arabidopsis Information Resource (TAIR): a model organism database providing a centralized, curated gateway to Arabidopsis biology, research materials and community. Nucleic Acids Res 31:224–228
Roberts JK, Pryor A (1995) Isolation of a flax (Linum usitatissimum) gene induced during susceptible infection by flax rust (Melampsora lini). Plant J 8:1–8
Rosen S, Skaletsky H (2000) Primer 3 on the WWW for general users and for biologist programmers. In: Krawtez S, Misener S (eds) Bioinformatics methods and protocols: methods in molecular biology. Humana, Totowa, NJ, pp 365–386
Schneider RW, Hollier CA, Whitham HK, Palm ME, McKemy JM, Hernandez JR, Levy L, DeVries-Patterson R (2005) First report of soybean rust caused by Phakopsora pachyrhizi in the continental United States. Plant Dis 89:774
Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville S, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci U S A 97:11655–11660
Sconyers LE, Kemerait RC, Brock J, Phillips DV, Jost PH, Sikora EJ, Gutierrez-Estrada A, Mueller JD, Marois JJ, Wright DL, Harmon CL (2006) Asian soybean rust development in 2005: A perspective from the Southeastern United States. Online. APSnet Feature, American Phytopathological Society, St. Paul, MN
Shah J (2005) Lipids, lipases, and lipid-modifying enzymes in plant disease resistance. Ann Rev Phytopathol 43:229–260
Singh BB, Gupta SC, Singh BD (1974) Sources of field resistance to rust [Phakopsora pachyrhizi] and yellow mosaic diseases of soybean. Indian J Genet Plant Breed 34:400–404
Tan YJ, Yu Z, Yang CY (1996) Soybean rust. China Agricultural Press, Beijing, China
Tomkins JP, Mahalingam R, Smith H, Goicoechea JL, Knap HT, Wing RA (1999) A bacterial artificial chromosome library for soybean PI 437654 and identification of clones associated with cyst nematode resistance. Plant Mol Biol 41:25–32
Tschanz AT, Wang TC, Tsai BY (1986) Recent advances in soybean rust research at Asian Vegetable Research and Development Center. In: Shanmugasundaram S, Sulberger EW (eds) Soybeans in tropical and subtropical cropping systems. Asian Vegetable Research and Development Center, Shanhua, Tainan, Taiwan, pp 237–245
Unni SL, Rune S, Pål S, Cathrine L (2007) Nitrogen deficiency enhances expression of specific MYB and bHLH transcription factors and accumulation of end products in the flavonoid pathway. Planta 225:1245–1253
van de Mortel M, Recknor JC, Graham MA, Nettleton D, Dittman JD, Nelson RT, Godoy CV, Abdelnoor RV, Almeida AMR, Baum TJ, Whitham SA (2007) Distinct biphasic mRNA changes in response to Asian soybean rust infection. Mol Plant-Microb Interact 20:887–899
Verica JA, Maximova SN, Strem MD, Carlson JE, Bailey BA, Guiltinan MJ (2004) Isolation of ESTs from cacao (Theobroma cacao L.) leaves treated with inducers of the defense response. Plant Cell Rep 23:404–413
Vucich V, Gasser C (1996) Novel structure of a high molecular weight FK506 binding protein from Arabidopsis thaliana. Mol Gen Genet 252:510–517
Webb CJ, Chan-Weiher C, Johnson DA (2008) Isolation of a novel family of genes related to 2-oxoglutarate-dependent dioxygenases from soybean and analysis of their expression during root nodule senescence. J Plant Physiol (in press). DOI 10.1016/j.jplph.2007.10.004
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Wu CH, Huang H, Nikolskaya A, Hu Z, Barker WC (2004) The iProClass integrated database for protein functional analysis. Comput Biol Chem 28:87–96
Wu CH, Apweiler R, Bairoch A, Natale DA, Barker WC, Boeckmann B, Ferro S, Gasteiger E, Huang H, Lopez R, Magrane M, Martin MJ, Mazumder R, O’Donovan C, Redaschi N, Suzek B (2006) The Universal Protein Resource (UniProt): an expanding universe of protein information. Nucleic Acids Res 34:D187–D191
Yang Y, Dudoit S, Luu P, Lin D, Peng V, Ngai J, Speed T (2002) Normalization of cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acid Res 30:e15
Zabala G, Zou J, Tuteja J, Gonzalez DO, Clough SJ, Vodkin LO (2006) Transcriptome changes in the phenylpropanoid pathway of Glycine max in response to Pseudomonas syringae infection. BMC Plant Biol 6:26
Zhulidov PA, Bogdanova EA, Shcheglov AS, Vagner LL, Khaspekov GL, Kozhemyako VB, Matz MV, Meleshkevitch E, Moroz LL, Lukyanov SA, Shagin DA (2004) Simple cDNA normalization using kamchatka crab duplex-specific nuclease. Nucleic Acids Res 32:e37
Zou J, Rodriguez-Zas S, Aldea M, Li M, Zhu J, Gonzalez DO, Vodkin LO, DeLucia E, Clough SJ (2005) Expression profiling soybean response to Pseudomonas syringae reveals new defense-related genes and rapid HR-specific downregulation of photosynthesis. Mol Plant-Microb Interact 18:1161–1174
Acknowledgments
We gratefully acknowledge Christine Stone and Craig Austin for maintenance and propagation of P. Pachyrhizi isolates and with the inoculations. We also would like to thank Hunter Beard for microarray slide spotting and technical expertise. This project was funded in part by the United Soybean Board as Projects 2229, 3217, and 4217 and supports the goals of the USDA National Strategic Plan for the Coordination and Integration of Soybean Rust Research. The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the US Department of Agriculture or the Agricultural Research Service of any product or service to exclusion of others that may be suitable.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Transcripts found to be differentially expressed by custom microarray analysis of immune vs. susceptible soybean leaves inoculated with P. pachryhizi and sampled at four times after inoculationa (DOC 1.05 mb)
Rights and permissions
About this article
Cite this article
Choi, J.J., Alkharouf, N.W., Schneider, K.T. et al. Expression patterns in soybean resistant to Phakopsora pachyrhizi reveal the importance of peroxidases and lipoxygenases. Funct Integr Genomics 8, 341–359 (2008). https://doi.org/10.1007/s10142-008-0080-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-008-0080-0