Abstract
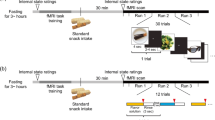
The goal of the present study was to investigate whether the psychophysical evaluation of taste stimuli using magnitude estimation influences the pattern of cortical activation observed with neuroimaging. That is, whether different brain areas are involved in the magnitude estimation of pleasantness relative to the magnitude estimation of intensity. fMRI was utilized to examine the patterns of cortical activation involved in magnitude estimation of pleasantness and intensity during hunger in response to taste stimuli. During scanning, subjects were administered taste stimuli orally and were asked to evaluate the perceived pleasantness or intensity using the general Labeled Magnitude Scale (Green et al., Chem Senses, 21(3), 323-334, 1996; Bartoshuk et al., Physiol Behav, 82(1), 109-114, 2004). Image analysis was conducted using Analysis of Functional NeuroImage software. Magnitude estimation of intensity and pleasantness shared common activations in the insula, rolandic operculum, and the medio-dorsal nucleus of the thalamus. Globally, magnitude estimation of pleasantness produced significantly more activation than magnitude estimation of intensity. Areas differentially activated during magnitude estimation of pleasantness versus intensity included, e.g., the insula, the anterior cingulate gyrus, and putamen, suggesting that different brain areas were recruited when subjects made magnitude estimates of intensity and pleasantness. These findings demonstrate significant differences in brain activation during magnitude estimation of intensity and pleasantness to taste stimuli. An appreciation for the complexity of brain response to taste stimuli may facilitate a clearer understanding of the neural mechanisms underlying eating behavior and overconsumption.


Similar content being viewed by others
References
Baliki MN, Geha PY, Apkarian AV (2009) Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol 101(2):875–887
Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM (2004) Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav 82(1):109–114
Benjamin RM, Burton H (1968) Projection of taste nerve afferents to anterior opercular–insular cortex in squirrel monkey (Saimiri sciureus). Brain Res 7(2):221–231
Bornstein WS (1940) Cortical representation of taste in man and monkey. II. The localization of the cortical taste area in man and a method of measuring impairment of taste in man. Yale J Biol Med 13:133–156
Cain WS, Gent J, Catalanotto FA, Goodspeed RB (1983) Clinical evaluation of olfaction. Am J Otolaryngol 4:252–256
Cerf-Ducastel B, Van De Moortele PF, MacLeod P, Le Bihan D, Faurion A (2001) Interaction of gustatory and lingual somatosensory perceptions at the cortical level in the human: a functional magnetic resonance imaging study. Chem Senses 26(4):371–383
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29(3):162–173
Critchley H, Daly E, Phillips M, Brammer M, Bullmore E, Williams S, Van Amelsvoort T, Robertson D, David A, Murphy D (2000) Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum Brain Mapp 9(2):93–105
de Araujo IE, Kringelbach ML, Rolls ET, Hobden P (2003a) Representation of umami taste in the human brain. J Neurophysiol 90(1):313–319
de Araujo IE, Kringelbach ML, Rolls ET, McGlone F (2003b) Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol 90(3):1865–1876
de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N (2003c) Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci 18(7):2059–2068
Elliott R, Dolan RJ, Frith CD (2000) Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex 10(3):308–317
Faurion A, Cerf B, Van De Moortele PF, Lobel E, Mac Leod P, Le Bihan D (1999) Human taste cortical areas studied with functional magnetic resonance imaging: evidence of functional lateralization related to handedness. Neurosci Lett 277(3):189–192
Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Clare S, Smith E (1999) The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 10(3):453–459
Gehring JR, Willoughby AR (2002) The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295(5563):2279–2282
Grabenhorst F, Rolls ET (2008) Selective attention to affective value alters how the brain processes taste stimuli. Eur J Neurosci 27(3):723–729
Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J (1996) Evaluating the 'Labeled Magnitude Scale' for measuring sensations of taste and smell. Chem Senses 21(3):323–334
Green BG, Shaffer GS, Gilmore MM (1993) A semantically-labeled magnitude scale of oral sensation with apparent ratio properties. Chemical Senses 18:683–702
Green E, Jacobson A, Haase L, Murphy C (2011) Reduced nucleus accumbens and caudate nucleus activation to a pleasant taste is associated with obesity in older adults. Brain Res 1386:109–117
Haase L, Green E, Jacobson A, Murphy C (2011) Males and females show differential brain activation to taste when hungry and sated in gustatory and reward areas. Appetite 57(2):421–434
Haase L, Cerf-Ducastel B, Murphy C (2009a) Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. NeuroImage 44:1008–1021
Haase L, Cerf-Ducastel B, Murphy C (2009b) The effect of stimulus delivery technique on perceived intensity functions for taste stimuli: implications for fMRI studies. Attention Percept Psychophys 71:1167–1173
Haase L, Cerf-Ducastel B, Buracas G, Murphy C (2007) On-line psychophysical data acquisition and event-related fMRI protocol optimized for the investigation of brain activation in response to gustatory stimuli. J Neurosci Methods 159(1):98–107
Hadland KA, Rushworth MF, Gaffan D, Passingham RE (2003) The anterior cingulate and reward-guided selection of actions. J Neurophysiol 89(2):1161–1164
Hariri AR, Bookheimer SY, Mazziotta JC (2000) Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport 11(1):43–48
Harris R, Davidson TM, Murphy C, Gilbert PE, Chen M (2006) Clinical evaluation and symptoms of chemosensory impairment: one thousand consecutive cases from the Nasal Dysfunction Clinic in San Diego. Am J Rhinol 20:101–108
Haruno M, Kawato M (2006) Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol 95(2):948–959
Hori Y, Minamimoto T, Kimura M (2009) Neuronal encoding of reward value and direction of actions in the primate putamen. J Neurophysiol 102(6):3530–3543
Jacobson A, Green E, Murphy C (2010) Age-related functional changes in gustatory and reward processing regions: an fMRI study. NeuroImage 53(2):602–620
Kinomura S, Kawashima R, Yamada K, Ono S, Itoh M, Yoshioka S, Yamaguchi T, Matsui H, Miyazawa H, Itoh H et al (1994) Functional anatomy of taste perception in the human brain studied with positron emission tomography. Brain Res 659(1–2):263–266
Kobayakawa T, Endo H, Ayabe-Kanamura S, Kumagai T, Yamaguchi Y, Kikuchi Y, Takeda T, Saito S, Ogawa H (1996) The primary gustatory area in human cerebral cortex studied by magnetoencephalography. Neurosci Lett 212(3):155–158
Kobayakawa T, Ogawa H, Kaneda H, Ayabe-Kanamura S, Endo H, Saito S (1999) Spatio-temporal analysis of cortical activity evoked by gustatory stimulation in humans. Chem Senses 24(2):201–209
Liberzon I, Taylor SF, Fig LM, Decker LR, Koeppe RA, Minoshima S (2000) Limbic activation and psychophysiologic responses to aversive visual stimuli. Interaction with cognitive task. Neuropsychopharmacology 23(5):508–516
Motta G (1959) I centri corticali del gusto. Bulletino delle Scienze Mediche 131:480–493
Murphy C, Schubert M, Cruickshanks K, Klein B, Klein R, Nondahl D (2002) Prevalence of olfactory impairment in older adults. JAMA: J Am Med Assoc 288:2307–2312
O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F (2001) Representation of pleasant and aversive taste in the human brain. J Neurophysiol 85(3):1315–1321
O'Doherty JP, Buchanan TW, Seymour B, Dolan RJ (2006) Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron 49(1):157–166
Penfield W, Faulk ME (1955) The insula. Further observations on its function. Brain 78(4):445–470
Pritchard TC, Hamilton RB, Morse JR, Norgren R (1986) Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol 244(2):213–228
Pritchard TC, Macaluso DA, Eslinger PJ (1999) Taste perception in patients with insular cortex lesions. Behav Neurosci 113(4):663–671
Rolls BJ, Rolls ET, Rowe EA, Sweeney K (1981) Sensory specific satiety in man. Physiol Behav 27(1):137–142
Rolls ET (1995) Central taste anatomy and neurophysiology. In: Doty RL (ed) Handbook of olfaction and gustation. Dekker, New York, pp 549–573
Rolls ET (2000) The orbitofrontal cortex and reward. Cereb Cortex 10(3):284–294
Rolls ET, Rolls BJ, Rowe EA (1983) Sensory-specific and motivation-specific satiety for the sight and taste of food and water in man. Physiol Behav 30(2):185–192
Rolls ET, Sienkiewicz ZJ, Yaxley S (1989) Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Eur J Neurosci 1(1):53–60
Scott TR (1981) Brainstem and forebrain involvement in the gustatory neural code, brain mechanisms of sensation. Wiley and Sons, New York, pp 177–196
Scott TR, Giza BK (2000) Issues of gustatory neural coding: where they stand today. Physiol Behav 69(1–2):65–76
Scott TR, Plata-Salaman CR, Smith VL, Giza BK (1991) Gustatory neural coding in the monkey cortex: stimulus intensity. J Neurophysiol 65(1):76–86
Scott TR, Yan J, Rolls ET (1995) Brain mechanisms of satiety and taste in macaques. Neurobiology 3:281–292
Scott TR, Yaxley S, Sienkiewicz ZJ, Rolls ET (1986) Gustatory responses in the frontal opercular cortex of the alert cynomolgus monkey. J Neurophysiol 56(3):876–890
Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T (2003a) Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron 39(4):701–711
Small DM, Jones-Gotman M, Dagher A (2003b) Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. NeuroImage 19(4):1709–1715
Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T (2003c) Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron 39:701–711
Spetter MS, Smeets PAM, de Graaf C, Viergever MA (2010) Representation of sweet and salty taste intensity in the brain. Chemical Senses 35(9):831–840. doi:10.1039/chemse/bjq093
Smith-Swintosky VL, Plata-Salaman CR, Scott TR (1991) Gustatory neural coding in the monkey cortex: stimulus quality. J Neurophysiol 66(4):1156–1165
Taylor SF, Phan KL, Decker LR, Liberzon I (2003) Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage 18(3):650–659
Veldheuizen M, Small DM (2011) Modality-specific neural effects of selective attention to taste and odor. Chem Senses 36(8):747–760
Wallis JD (2007) Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci 30:31–56
Woods AT, Lloyd DM, Kuenzel J, Poliakoff E, Dijksterhuis GB, Thomas A (2011) Expected taste intensity affects response to sweet drinks in primary taste cortex. Neuroreport 22(8):365–369
Yaxley S, Rolls ET, Sienkiewicz ZJ (1990) Gustatory responses of single neurons in the insula of the macaque monkey. J Neurophysiol 63(4):689–700
Zald DH, Hagen MC, Pardo JV (2002) Neural correlates of tasting concentrated quinine and sugar solutions. J Neurophysiol 87(2):1068–1075
Zald DH, Lee JT, Fluegel KW, Pardo JV (1998) Aversive gustatory stimulation activates limbic circuits in humans. Brain 121(Pt 6):1143–1154
Zatorre RJ, Jones-Gotman M, Rouby C (2000) Neural mechanisms involved in odor pleasantness and intensity judgments. Neuroreport 11(12):2711–2716
Acknowledgments
This study was funded by NIH grants R01AG04085, R01DC02064 and R03DC051234. We thank Dr. Richard Buxton for fMRI expertise.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cerf-Ducastel, B., Haase, L. & Murphy, C. Effect of Magnitude Estimation of Pleasantness and Intensity on fMRI Activation to Taste. Chem. Percept. 5, 100–109 (2012). https://doi.org/10.1007/s12078-011-9109-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12078-011-9109-1