Abstract
To have uniform nanoparticles individually dispersed on substrate before single-walled carbon nanotubes (SWNTs) growth at high temperature is the key for controlling the diameter of the SWNTs. In this letter, a facile approach to control the diameter and distribution of the SWNTs by improving the dispersion of the uniform Fe/Mo nanoparticles on silicon wafers with silica layer chemically modified by 1,1,1,3,3,3-hexamethyldisilazane under different conditions is reported. It is found that the dispersion of the catalyst nanoparticles on Si wafer surface can be improved greatly from hydrophilic to hydrophobic, and the diameter and distribution of the SWNTs depend strongly on the dispersion of the catalyst on the substrate surface. Well dispersion of the catalyst results in relatively smaller diameter and narrower distribution of the SWNTs due to the decrease of aggregation and enhancement of dispersion of the catalyst nanoparticles before growth. It is also found that the diameter of the superlong aligned SWNTs is smaller with more narrow distribution than that of random nanotubes.
Similar content being viewed by others
1 Introduction
Since the first report by Iijima in 1991 [1], carbon nanotubes (CNTs) have attracted tremendous attentions both for fundamental research and potential applications in many fields [2–4]. Especially, the single-wall carbon nanotubes (SWNTs) are much more interesting for scientists due to their unique one-dimensional structure, mechanical, and electrical properties [5]. SWNTs consist of a single graphite sheet seamlessly rolled into cylindrical tube. They can be grown by several methods including arc discharge [6], laser ablation [7], and chemical vapor deposition (CVD) [8]. Among these methods, CVD is the most promising method for low-cost and large-scale production of highly pure nanotubes. SWNTs can be grown in high temperature using carbon-containing molecules such as methane, ethane, or carbon monoxide [9] as carbon sources with the help of catalytic particles, usually metal nanoparticles (NPs) such as Fe, Co, Ni, and their alloys. In most cases, only SWNTs with different chiralities can be generated, this is not beneficial for their applications particularly for electronic devices application because the electrical properties of the SWNTs depend not only on their structure (chirality) [10, 11] but also on their diameter [12]. Therefore, how to take effective control of the chirality and diameter is still a goal of current research [13–25]. Basically, the diameter of SWNTs depends on the size of the catalyst. The catalyst can be pre-obtained by either chemical method (nanoparticles in solution) or physical methods (such as arc plasma or sputtering deposition or thermal treatment of thin film of catalyst on substrate). How to prepare uniform catalyst size is the key for controlling the diameter of SWNTs. In the past decades, many efforts have been put on to control the catalyst size for the control of the SWNTs diameter. For example, Dai and Pfefferle got uniform diameter SWNTs using polyamidoamine dendrimer [26] or MCM-41 [27] as catalyst carriers, respectively. Hyon et al. reported one way to control the diameter of the SWNTs by Langmuir–Blodgett film [28]. Jie used identical metal-containing molecular nanoclusters as catalysts and investigated the relationship between the diameter of SWNTs and carbon feeding [29, 30]. Size-separated ferritin-based Fe catalyst [31], inorganic oxides nanoparticles with high melting point such as SiO2, TiO2, Al2O3 are also used as catalysts for growing SWNTs [32–34]. Arc plasma deposition [35] or floating catalyst method [36] are applied to control the size and density of the catalysts for growing either vertically aligned SWNT forest or narrowing the SWNT diameter distribution. Although significant progress has been made, to develop a facile technology to control accurately the diameters of SWNTs and to understand the relationship between the diameters of SWNTs and the catalyst size still remains as a challenge. It is believed that uniform NPs individually dispersed on the substrate before CNTs growth at high temperature is one of the keys for controlling the diameters of the SWNTs. However, catalyst NPs particularly metal NPs tend to aggregate at high temperature. In this article, we report a facile approach to control the diameter of SWNTs by improving the dispersion of the Fe/Mo NPs on silicon wafers with different surface properties by chemical modification with 1,1,1,3,3,3-hexamethyldisilazane (HMDS).
2 Experimental
In our experiment, silicon wafer with 200-nm thick silica on top as substrate was used as substrate. The substrates were modified by 1,1,1,3,3,3-hexamethyldisilazane. The overall synthetic procedure was described in Scheme 1. To start with, all wafers were first cleaned by piranha solution (a mixture of 98 % H2SO4 and 30 % H2O2 at 7:3 v/v) for about 30 min, followed by thorough rinsing with deionized water for several times. Then, 10 mL of HMDS was added into a steel chamber (300 mL in volume). The wafers were then put into the chamber at room temperature and 150 °C for different times, respectively (without modified, modified for 1 min at room temperature, and modified for 30 min at 150 °C). The modification of the substrate surface was monitored by contact angle measurement using water as (EASYDROP Contact Angle Measuring System, Krüss Company).
The uniform Fe/Mo NPs were synthesized by thermal decomposition of Fe(CO)5/Mo(CO)6 under the protection of the surfactant according to the Ref. [37]. The dispersion of the Fe/Mo NPs was achieved by dipping the modified and without modified wafers into the hexane solution containing Fe/Mo NPs. After the solvent was evaporated, the dried wafers were heated in hydrogen (H2) at 900 °C. When the furnace was cooled down to room temperature, the distribution of particles was characterized by atomic force microscopy on tapping mode (AFM: Nano Scope III).
CVD method was used for SWNTs growth. Typically, the wafer with the catalyst was put in a quartz tube. Then the quartz tube was put into a tube furnace and heated in argon (Ar). Then after the furnace was heated up to 900 °C under the mixture of Ar and H2, Ar was turned off and methane was introduced into the tube (ratio of CH4/H2 = 4:1). After 15 min, methane and H2 were turned off and the quartz tube was cooled to room temperature under the Ar. The samples were characterized by AFM and scanning electron microscope (SEM: FEI NanoSEM at 10 kV) and Raman spectroscopy (Dilor Raman spectrometer with triple spectrograph at 514.5 nm excitation wavelength with an Ar-ion laser source at a power of 120 mW). The diameter of the SWNTs was measured by AFM.
3 Results and Discussion
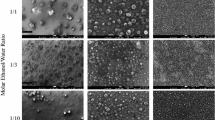
At room temperature, the contact angle of the bare Si/SiO2 substrate was measured to be about 32° (Fig. 1a). But after the substrate was exposed to HMDS in a steel chamber, the contact angle of the substrate was found to increase with the extension of time (Fig. 1a). When the contact angle reached to 90°, it would not change with the time. However, when steel chamber was put in the oven at 150 °C, the contact angle of the substrate will change with the time until the contact angle reaches about 112° (Fig. 1b). In order to study the relationship of the surface property of the substrates and the dispersion of the catalyst NPs, three substrates were selected for comparison: (a) bare substrate (contact angle is 32°); (b) the substrate modified for 1 min at room temperature (contact angle is 71°); (c) the substrate modified for 30 min at 150 °C (contact angle is 110°). The picture of the water droplet is given in Fig. 1c–e, respectively. The catalyst used for the growth of SWNTs is uniform Fe/Mo NPs wrapped by organic surfactant in the n-heptane. The diameter of the NPs was measured to be 3.8 ± 0.8 nm according to TEM observation (Fig. 2). After dispersion of the solution containing Fe/Mo NPs on the substrate, the substrate was treated under Ar at 300 °C to remove the organic surfactant. Figure 3a shows AFM images and height measurement of the Fe/Mo NPs after dispersion on the bare substrate. It was easy to note that some NPs aggregated because of the hydrophilic surface of the bare wafer. The corresponding statistical size distribution of the NPs ranging from 3.0 to 12.0 nm is given in Fig. 3a. The main size was 5.0–9.0 nm, indicating the aggregation of the Fe/Mo NPs on the bare substrate surface. The dispersion of the Fe/Mo NPs on the substrate can be improved a lot after the modification by HMDS because the substrate became hydrophobic. When the contact angle of the substrate is 71° and 110°, the main size of the Fe/Mo NPs is 3.0–6.0 nm (Fig. 3b) and 2.0–5.0 nm (Fig. 3c), respectively. From this result, it is obvious that the aggregation degree of the Fe/Mo NPs on the substrate surface can be decreased by modifying the substrate from hydrophilic to hydrophobic. As the size of the Fe/Mo NPs is 3.8 ± 0.8 nm, most of the NPs can be dispersed on the substrate separately when the surface becomes fully hydrophobic.
It is well known that the diameters of CNTs depend strongly on the catalyst size. Uniform Fe/Mo NPs have been widely used as active catalyst for the growth of SWNTs on substrate [9]. Changing the wettability of the SiO2/Si substrate has been applied to control the density of the nanotubes and pattern of the nanotubes [38]. By improving the dispersion of the uniform Fe/Mo NPs on the substrate surface a better control for the uniformity of the SWNTs diameters could be expected. Figure 4 presents the AFM images and the diameter distribution of the SWNTs growing on the substrates with or without chemical modification using methane as carbon source. Figure 4a is the AFM image and height measurement of an individual SWNT and the diameter distribution of the SWNTs, as well on the bare substrate. From the diameter distribution, the main diameters of the nanotubes was 0.8–3.3 nm (the main diameter of the SWNTs is 1.75 nm). When the substrate was modified by HMDS, the diameters of the SWNTs were found to decrease and the size distribution became narrow. Figure 4b, c gives the AFM images and the height of the individual SWNT and size distribution. The main diameters of the SWNTs is 0.8–2.8 nm (the main diameter of the SWNTs is 1.50 nm) for the substrate modified by HMDS for 1 min at room temperature (Fig. 4b) and 1.1–2.0 nm (the main diameter of the SWNTs is 1.46 nm) for the substrate modified by HMDS for 30 min at 150 °C, respectively. This results indicate that the hydrophobic surface will reduce the aggregation and improve the dispersion of the catalytic NPs on the surface because the uniform Fe/Mo NPs was dispersed in hydrophobic solvent (n-heptane), thus reducing the diameter of the nanotubes and narrowing the diameter distribution. This approach as-demonstrated is simply changing the hydrophobicity, matching the solvent in which the catalyst NPs are dispersed. This method should be a simple and facile one compared with other methods applied in controlling the diameter of SWNTs [35, 39–42].
As we have demonstrated that when “fast-heating” CVD is used superlong well-aligned SWNTs arrays can be generated and “kite mechanism” has been proposed to describe how the long SWNTs can grow and how they can be aligned (gas flow guidance) [43–45]. What is interesting to find is that the diameter of the long-oriented SWNTs was smaller with more narrower distribution than that of random short SWNTs. Figure 5a, b is the SEM images of the superlong well-aligned SWNT arrays, and Fig. 5c–e is the AFM image, height measurement, and diameter distribution of the long-aligned SWNTs on the substrate modified by HMDS for 1 min at room temperature. The diameter of the SWNTs ranges from 0.5 to 2.5 nm (the main diameter is 1.26 nm). Comparing with the random SWNTs (Fig. 4) and well-aligned SWNT array (Fig. 5c–e), it was easy to note that the diameter of the aligned SWNTs is larger than that of random one and the diameter distribution of the aligned SWNTs is much narrower that that of the random nanotubes on bare substrate (Fig. 4a), and even narrower than that of the nanotubes on modified substrate (Fig. 4b, c). As we have demonstrated the growth of the superlong nanotubes under “fast-heating” CVD condition is tip-growth mechanism (kite mechanism) and the orientation of the nanotubes is controlled by the gas flow [44]. The “fast-heating” process limits the aggregation of the catalyst NPs on the substrate and the catalyst with small size should be relatively easy to grow in tip-growth mechanism because of the relative weak interaction with the substrate surface. Furthermore, the aligned SWNTs growth in the gas flow also makes the nanotubes much cleaner than that of the random nanotubes which locates the catalyst NPs area.
4 Conclusions
In this study, we successfully improved the Fe/Mo NPs dispersion and reduced their aggregation on substrate surface by modifying the substrate from hydrophilic to hydrophobic. Different diameters and distribution of SWNTs can be generated by using different size NPs based on CVD. For fully hydrophobic surface, Fe/Mo NPs can be possibly separate individual dispersion, and high-yield SWNTs with narrow size distribution SWNTs can be obtained. It is also found that the diameter of the superlong aligned SWNTs is smaller with more narrow distribution than that of random nanotubes due to the tip-growth mechanism for oriented nanotubes. Results further confirm that the diameter of SWNTs has a closed relationship with the size of catalyst and also provide an effective way to control the diameter and size distribution of SWNTs by improving the dispersion of the uniform catalyst NPs on surface.
References
S. Iijima, Helical microtubules of graphitic carbon. Nature 354, 56–58 (1991). doi:10.1038/354056a0
J. Li, W. Yue, Z. Guo, Y. Yang, X. Wang, A.A. Syed, Y. Zhang, Unique characteristics of vertical carbon nanotube field-effect transistors on silicon. Nano-Micro Lett. 6(3), 287–292 (2014). doi:10.5101/nml140031a
M.F.L. De Volder, S.H. Tawfick, R.H. Baughman, A.J. Hart, Carbon nanotubes: present and future commercial applications. Science 339(6119), 535–539 (2013). doi:10.1126/science.1222453
Q. Wei, Y. Liu, L. Zhang, S. Huang, Growth and formation mechanism of branched carbon nanotubes by pyrolysis of iron(II) phthalocyanine. Nano-Micro Lett. 5(2), 124–128 (2013). doi:10.5101/nml.v5i2.p124-128
M.S. Dresselhaus, G. Dresselhaus, P. Avouris, Carbon Nanotubes Synthesis, Structure, Properties, and Applications (Springer, New York, 2001)
T.W. Ebbesen, P.M. Ajayan, Large-scale synthesis of carbon nanotubes. Nature 358, 220–222 (1992). doi:10.1038/358220a0
T. Guo, P. Nikolaev, A.G. Rinzler, D. TomBnek, D.T. Colbert, R.E. Smalley, Self-assembly of tubular fullerenes. J. Phys. Chem. B 99(27), 10694–10699 (1995). doi:10.1021/j100027a002
M. Su, B. Zheng, J. Liu, A scalable CVD method for the synthesis of single walled carbon nanotubes with high catalyst productivity. Chem. Phys. Lett. 322(5), 321–324 (2000). doi:10.1016/S0009-2614(00)00422-X
S.M. Huang, X.Y. Cai, J. Liu, Growth of millimeter-long and horizontally aligned SWNT on flat substrates. J. Am. Chem. Soc. 125(19), 5636–5637 (2003). doi:10.1021/ja034475c
J.W.G. WildÖer, L.C. Venema, A.G. Rinzler, R.E. Smalley, C. Dekker, Electronic structure of atomically resolved carbon nanotubes. Nature 391, 59–62 (1998). doi:10.1038/34139
T.W. Odom, J.L. Huang, P. Kim, C.M. Lieber, Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 391, 62–64 (1998). doi:10.1038/34145
H.S. Cheng, A.C. Copper, G.P. Pez, M.K. Kistov, P. Piotrowski, S.J. Stuart, Molecular dynamics simulations on the effects of diameter and chirality on hydrogen adsorption in single walled carbon nanotubes. J. Phys. Chem. B 109(9), 3780–3786 (2005). doi:10.1021/jp045358m
X. Tu, S. Manohar, A. Jagota, M. Zheng, DNA sequence motifs for structure-specific recognition and separation of carbon nanotubes. Nature 460, 250–253 (2009). doi:10.1038/nature08116
H. Liu, T. Tanaka, Y. Urabe, H. Kataura, High-efficiency single-chirality separation of carbon nanotubes using temperature-controlled gel chromatography. Nano Lett. 13(5), 1996–2003 (2013). doi:10.1021/nl400128m
G. Hong, M. Zhou, R. Zhang, S. Hou, W. Choi et al., Separation of metallic and semiconducting single-walled carbon nanotube arrays by scotch tape. Angew. Chem. Int. Ed. 50(30), 6819–6823 (2011). doi:10.1002/anie.201101700
Q. Wang, H. Wang, L. Wei, S.-W. Yang, Y. Chen, Reactive sites for chiral selective growth of single-walled carbon nanotubes: a DFT study of Ni55–Cn complexes. J. Phys. Chem. A 116(47), 11709–11717 (2012). doi:10.1021/jp308115f
D. Dutta, W.-H. Chiang, R.M. Sankaran, V.R. Bhethanabotla, Epitaxial nucleation model for chiral-selective growth of single-walled carbon nanotubes on bimetallic catalyst surfaces. Carbon 50(10), 3766–3773 (2012). doi:10.1016/j.carbon.2012.03.051
M. He, P.V. Fedotov, E.D. Obraztsova, V. Viitanen, J. Sainio et al., Chiral-selective growth of single-walled carbon nanotubes on stainless steel wires. Carbon 50(11), 4294–4297 (2012). doi:10.1016/j.carbon.2012.05.007
W.-H. Chiang, R.M. Sankaran, Linking catalyst composition to chirality distributions of as-grown single-walled carbon nanotubes by tuning Ni(x)fe(1-x) nanoparticles. Nat. Mater. 8, 882–886 (2009). doi:10.1038/nmat2531
S.M. Bachilo, L. Balzano, J.E. Herrera, F. Pompeo, D.E. Resasco, R.B. Weisman, Narrow (n,m)-distribution of single-walled carbon nanotubes grown using a solid supported catalyst. J. Am. Chem. Soc. 125(37), 11186–11187 (2003). doi:10.1021/ja036622c
B. Liu, W. Ren, S. Li, C. Liu, H.M. Cheng, High temperature selective growth of single-walled carbon nanotubes with a narrow chirality distribution from a CoPt bimetallic catalyst. Chem. Commun. 48(18), 2409–2411 (2012). doi:10.1039/c2cc16491d
M. He, A.I. Chernov, P.V. Fedotov, E.D. Obraztsova, J. Sainio et al., Predominant (6,5) single-walled carbon nanotube growth on a copper-promoted iron catalyst. J. Am. Chem. Soc. 132(40), 13994–13996 (2010). doi:10.1021/ja106609y
W.-H. Chiang, M. Sakr, X.P.A. Gao, R.M. Sankaran, Nanoengineering NixFe1-x catalysts for gas-phase, selective synthesis of semiconducting single-walled carbon nanotubes. ACS Nano 3(12), 4023–4032 (2009). doi:10.1021/nn901222t
M. He, H. Jiang, B. Liu, P.V. Fedotov, A.I. Chernov et al., Chiral-selective growth of single-walled carbon nanotubes on lattice-mismatched epitaxial cobalt nanoparticles. Sci. Rep. 3, 1460 (2013). doi:10.1038/srep01460
F. Yang, X. Wang, D. Zhang, J. Yang, D. Luo et al., Chirality-specific growth of single-walled carbon nanotubes on solid alloy catalysts. Nature 510, 522–524 (2014). doi:10.1038/nature13434
H.C. Choi, W. Kim, D. Wang, H.J. Dai, Delivery of catalytic metal species onto surfaces with dendrimer carriers for the synthesis of carbon nanotubes with narrow diameter distribution. J. Phys. Chem. B 106(48), 12361–12365 (2002). doi:10.1021/jp026421f
U.D. Ciuparu, Y. Chen, S. Lim, G.L. Haller, L. Pfefferle, Uniform diameter single-walled carbon nanotubes catalytically grown in cobalt-Incorporated MCM-41. J. Phys. Chem. B 180(2), 503–507 (2004). doi:10.1021/jp036453i
S.J. Han, T.Y. Yu, J. Park, B. Koo, J. Joo, T. Hyeon, Diameter-controlled synthesis of discrete and uniform-sized single-walled carbon nanotubes using monodisperse iron oxide nanoparticles embedded in zirconia nanoparticle arrays as catalysts. J. Phys. Chem. B 108(24), 8091–8095 (2004). doi:10.1021/jp037634n
L. An, J.M. Owens, L.E. McNeil, J. Liu, Synthesis of nearly uniform single-walled carbon nanotubes using identical metal-containing molecular nanoclusters as catalysts. J. Am. Chem. Soc. 124(46), 13688–13689 (2002). doi:10.1021/ja0274958
C.G. Lu, J. Liu, Controlling the diameter of carbon nanotubes in chemical vapor deposition method by carbon feeding. J. Phys. Chem. B 110(41), 20254–20257 (2006). doi:10.1021/jp0632283
L. Durrer, J. Greenwald, T. Helbling, M. Muoth, R. Riek, C. Hierold, Narrowing SWNT diameter distribution using size-separated ferritin-based Fe catalysts. Nanotechnology 20(35), 355601–355607 (2009). doi:10.1088/0957-4484/20/35/355601
S. Huang, Q. Cai, J. Chen, Q. Yong, L. Zhang, Catalyst-free growth of SWNTs on substrate. J. Am. Chem. Soc. 131(6), 2094–2095 (2009). doi:10.1021/ja809635s
Y. Chen, J. Zhang, Diameter controlled growth of single-walled carbon nanotubes from SiO2 nanoparticles. Carbon 49(10), 3316–3324 (2011). doi:10.1016/j.carbon.2011.04.016
Q. Cai, Y. Hu, Y. Liu, S. Huang, Growth of carbon nanotubes from titanium dioxide nanoparticles. Appl. Surf. Sci. 258(20), 8019–8025 (2012). doi:10.1016/j.apsusc.2012.04.161
G. Chen, Y. Seki, H. Kimura, S. Sakurai, M. Yumura, K. Hata, D.N. Futaba, Diameter control of single-walled carbon nanotube forests from 1.3–3.0 nm by arc plasma deposition. Sci. Rep. 4, 3804–3808 (2014). doi:10.1038/srep03804
Q. Liu, W. Ren, Z. Chen, D. Wang, B. Liu, F. Li, H. Cong, H. Chen, B. Yu, Diameter-selective growth of single-walled carbon nanotubes with high quality by floating catalyst method. ACS Nano 2(8), 1722–1728 (2008). doi:10.1021/nn8003394
Y. Li, J. Liu, Y.Q. Wang, Z.L. Wang, Preparation of monodispersed Fe–Mo nanoparticles as the catalyst for CVD synthesis of carbon nanotubes. Chem. Mater. 13(3), 1008–1014 (2001). doi:10.1021/cm000787s
R. Xiang, T. Wu, E. Einarsson, Y. Suzuki, Y. Murakami, J. Shiomi, S. Maruyama, High-precision selective deposition of catalyst for facile localized growth of single-walled carbon nanotubes. J. Am. Chem. Soc. 131(30), 10344–10345 (2009). doi:10.1021/ja902904v
G. Zhong, J.H. Warner, M. Fouquet, A.W. Robertson, B. Chen, J. Robertson, Growth of ultrahigh density single-walled carbon nanotube forests by improved catalyst design. ACS Nano 6(4), 2893–2903 (2012). doi:10.1021/nn203035x
W. Song, C. Jeon, Y.S. Kim, Y.T. Kwon, D.S. Jung et al., Synthesis of bandgap-controlled semiconducting single-walled carbon nanotubes. ACS Nano 4(2), 1012–1018 (2010). doi:10.1021/nn901135b
R. Xiang, E. Einarsson, Y. Murakami, J. Shiomi, S. Chiashi, Z. Tang, S. Maruyama, Diameter modulation of vertically aligned single-walled carbon nanotubes. ACS Nano 6(8), 7472–7479 (2012). doi:10.1021/nn302750x
Y. Tian, M.Y. Timmermans, S. Kivistö, A.G. Nasibulin, Z. Zhu, H. Jiang, O.G. Okhotnikov, E.I. Kauppinen, Tailoring the diameter of single-walled carbon nanotubes for optical applications. Nano Res. 4(8), 807–815 (2011). doi:10.1007/s12274-011-0137-6
S. Huang, X. Cai, J. Liu, Growth of millimeter-long and horizontally aligned SWNT on flat substrates. J. Am. Chem. Soc. 125(19), 5636–5637 (2003). doi:10.1021/ja034475c
S. Huang, B. Maynor, J. Liu, Ultralong, well-aligned single-walled carbon nanotube architectures on surfaces. Adv. Mater. 15(19), 1651–1654 (2003). doi:10.1002/adma.200305203
S. Huang, M. Woodson, J. Liu, R. Smalley, Growth mechanism of oriented long single-walled carbon nanotubes using “fast-heating” chemical vapor deposition process. Nano Lett. 4(6), 1025–1028 (2004). doi:10.1021/nl049691d
Acknowledgment
The author would like to appreciate the partially financial support from NSFC (21173159, 51420105002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chen, J., Xu, X., Zhang, L. et al. Controlling the Diameter of Single-Walled Carbon Nanotubes by Improving the Dispersion of the Uniform Catalyst Nanoparticles on Substrate. Nano-Micro Lett. 7, 353–359 (2015). https://doi.org/10.1007/s40820-015-0050-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40820-015-0050-8