Articles
Article Tools
Stats or Metrics
Article
Review Article
Exp Neurobiol 2018; 27(3): 139-154
Published online June 30, 2018
https://doi.org/10.5607/en.2018.27.3.139
© The Korean Society for Brain and Neural Sciences
The Emerging Concept of Intrinsic Plasticity: Activity-dependent Modulation of Intrinsic Excitability in Cerebellar Purkinje Cells and Motor Learning
Hyun Geun Shim1,2, Yong-Seok Lee1,2,3 and Sang Jeong Kim1,2,3*
1Department of Physiology, Seoul National University College of Medicine, Seoul 03080, 2Department of Biomedical Science, Seoul National University College of Medicine, Seoul 03080, 3Neuroscience Research Institute, Seoul National University College of Medicine, Seoul 03080, Korea
Correspondence to: *To whom correspondence should be addressed.
TEL: 82-2-740-8229, FAX: 82-2-763-9667
e-mail: sangjkim@snu.ac.kr
Abstract
What is memory? How does the brain process the sensory information and modify an organism's behavior? Many neuroscientists have focused on the activity- and experience-dependent modifications of synaptic functions in order to solve these fundamental questions in neuroscience. Recently, the plasticity of intrinsic excitability (called intrinsic plasticity) has emerged as an important element for information processing and storage in the brain. As the cerebellar Purkinje cells are the sole output neurons in the cerebellar cortex and the information is conveyed from a neuron to its relay neurons by forms of action potential firing, the modulation of the intrinsic firing activity may play a critical role in the cerebellar learning. Many voltage-gated and/or Ca2+-activated ion channels are involved in shaping the spiking output as well as integrating synaptic inputs to finely tune the cerebellar output. Recent studies suggested that the modulation of the intrinsic excitability and its plasticity in the cerebellar Purkinje cells might function as an integrator for information processing and memory formation. Moreover, the intrinsic plasticity might also determine the strength of connectivity to the sub-cortical areas such as deep cerebellar nuclei and vestibular nuclei to trigger the consolidation of the cerebellar-dependent memory by transferring the information.
Graphical Abstract
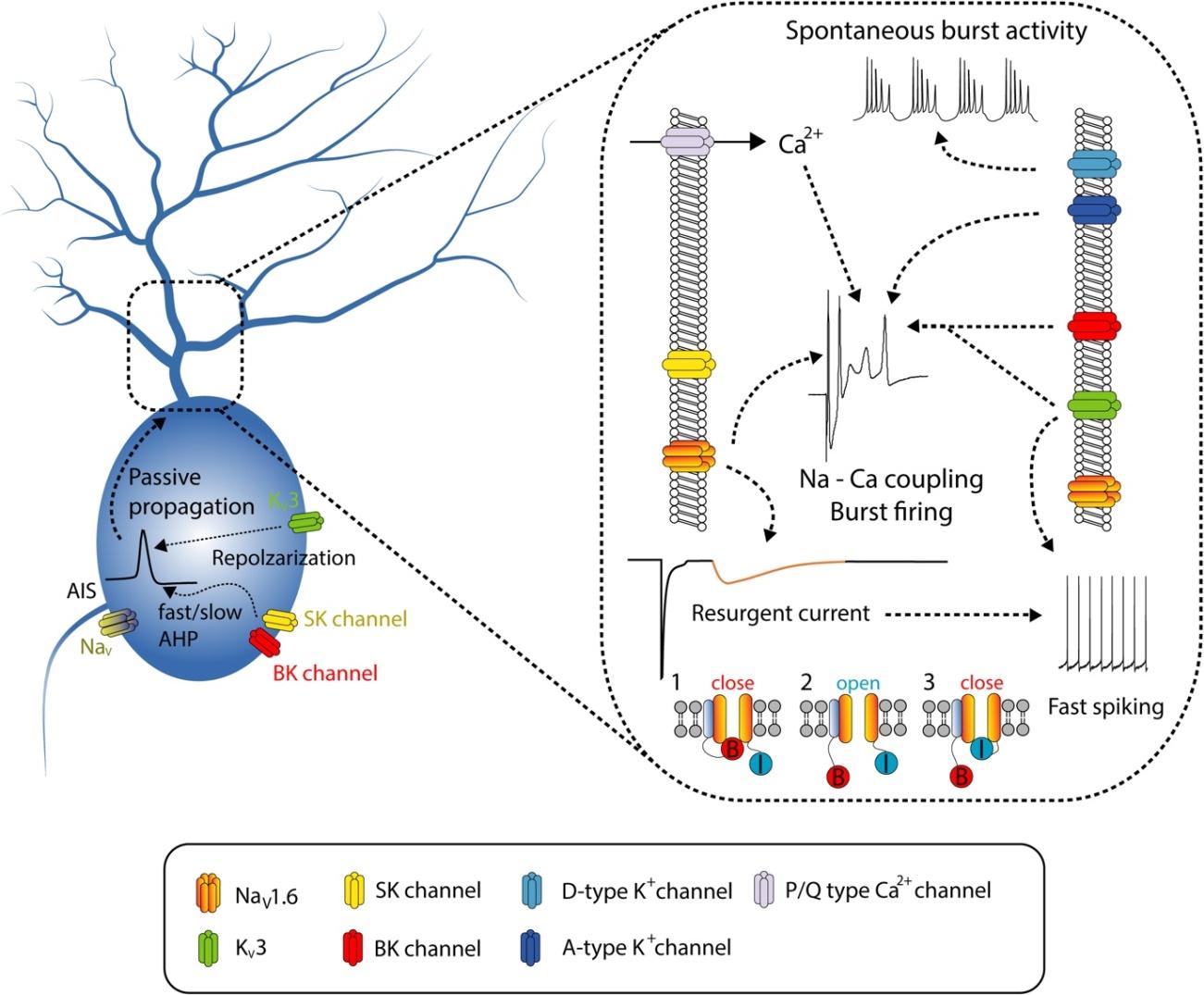
Keywords: Cerebellum, Purkinje cells, Excitability, Ion channels, Neuronal plasticity, Learning
INTRODUCTION
Since Hebb's rule was proposed, many neuroscientists have focused on the plastic changes in the synaptic neurotransmission within the given synapses such as long-term potentiation and depression (LTP and LTD, respectively) [1,2,3,4,5]. These persistent alterations of synaptic strength have been suggested to be a cellular basis of memory storage in the brain, which have been supported by the experimental observations in which experience- and use-dependent modulation of the synaptic function are exhibited by certain forms of behavioral training [4,6,7]. There is, however, accumulating evidence supporting the idea that information storage may also involve the activity-dependent modulation of neuronal intrinsic excitability (intrinsic plasticity) in addition to synaptic plasticity [8,9,10,11,12]. The intrinsic plasticity is not confined to the single synapse but accompanies non-synaptic and global changes [13,14]. Therefore, the incongruity between synaptic and intrinsic plasticity may give rise to a controversy of which the global changes of neuronal excitability would seemingly distort the experience-dependent synaptic plasticity. Notably, synapse-specific and non-specific modifications synergistically contribute to the information processing and memory storage in the defined circuitry [13,15,16,17]. When the synaptic plasticity occurs, several voltage-gated ion channels, which are related to the modulation of neuronal excitability, are endowed with activity-dependent up- or down-regulation [18,19,20,21]. The activity-dependent modulation of ion channels regulates not only intrinsic excitability but also a dendritic integration of the synaptic inputs [22,23]. The intrinsic plasticity determines the net output of neurons by integrating the synaptic inputs and consecutively translating them into the action potential (AP) firing. Given that information is conveyed from a neuron (presynaptic) to its following neuron (postsynaptic) by AP firing, synergies between synaptic and intrinsic plasticity would play a role in maximizing information processing such as encoding, transfer and storage.
The inhibitory principal neurons in the cerebellar cortex, the cerebellar Purkinje cells (PCs) integrate excitatory and inhibitory inputs from widely spread dendrite branches. Various sensory information from the pre-cerebellar region and spinal cord project into the cerebellum through the mossy fiber (MF) which forms synapses with the cerebellar granule cells providing excitatory synaptic inputs into the PCs through its axon fibers, parallel fibers (PFs). Cerebellar PCs integrate the sensory information from the PF and then provide inhibitory instructive signals to the neurons in the vestibular nuclei (VN) and/or the deep cerebellar nuclei (DCN) in order to generate motor output. This synaptic gain is modulated in an activity-dependent manner, which has long been considered as the cellular mechanism of cerebellum-dependent motor learning [12,24,25,26,27]. In addition to PF inputs, cerebellar PCs receive the other excitatory synaptic inputs from inferior olivary neuron axon fiber, climbing fiber (CF), encoding the feedback error signal corresponding to performances [28,29,30,31]. In order to control goal-directed movement, this sensory feedback of error signals dynamically regulates the cerebellar output [32,33]. Indeed, the CF inputs onto the cerebellar PCs are regarded as the instructive signals in the cerebellar plasticity as the PF-PC synaptic plasticity is guided by timing rules between PF and CF activation [34,35]. When the CF inputs are conjunctive and paired with PF inputs, the excitatory synaptic transmission within PF-PC synapses is attenuated, called PF-PC long-term depression (LTD). In contrast, repetitive and strong PF electrical stimulation is found to induce long-term potentiation (LTP) at this synapses when CF inputs are omitted or non-paired [36]. PF-PC synaptic plasticity, in fact, is the heterosynaptic plasticity guided depending on the timing rules between PF and CF activation. The performance error signals are conveyed by CF to re-compute the motor signal from PCs, enabling finely tuned motor coordination through determining the cerebellar cortical activity. Many implications in cerebellar motor learning have suggested that the bidirectional plasticity of PF-PC synapses may be selectively engaged in specific behavioral paradigms [37,38]. In spite of abundant studies on PF-PC LTD/LTP associated with cerebellum dependent behaviors, it has been less elucidated how the net output of the cerebellar PCs is regulated in an activity-dependent manner. Since the cerebellar PCs are the sole output of the cerebellar cortex, the plasticity of the intrinsic excitability in the neurons might play a pivotal role in the modulation of cerebellar motor behavior and learning. In this review, we first cover the ion channels regulating the spiking activity of the cerebellar PCs and the cellular mechanisms of the plastic changes in excitability. Furthermore, we discuss the physiological significance of the intrinsic plasticity and how the synergies between synaptic and intrinsic plasticity contribute to behavioral outcomes.
ION CHANNELS AND SPIKING ACTIVITY OF THE CEREBELLAR PCs
The intrinsic excitability is influenced by the conductance of voltage-gated ion channels generating ionic current carried by Na+ and K+ ions (
Voltage-gated Na+ channels (NaV) are involved in determining active properties of neurons including voltage threshold for generating AP at the axon hillock and amplitude of AP spike [40,41,42]. In the cerebellar PCs, various subtypes of voltage-gated Na+ channels are expressed, for instance, NaV1.1, NaV1.2 and NaV1.6 have been observed in rodent PCs [45,46,47,48,49]. Observation of [Na+]i changes and electrophysiological recordings via outside-out patch clamp configuration have revealed that Na+ spikes are generated within the somatic area of PC and then passively spread into the dendrites [49]. Despite some subtypes of NaV and changes in [Na+]i in the PC dendrites were observed, backpropagation of AP into the dendrite from soma is absent, suggesting that the [Na+]i influx and subtypes of Na+ channels expressed in dendrites are not sufficient to generate AP in PC dendrites. In addition, electrophysiological recordings of
Among subtypes of NaV, NaV1.6 shows a distinct feature, which contributes to transient and resurgent components, but not to persistent components (Table 1). NaV1.6 was remarkably observed in the dendritic area in the cerebellar PCs [51]. The resurgent
K+ channels are another major players in determining the excitability of the neurons. As molecular techniques have been advanced, various subtypes of K+ channels and their gating properties have been characterized [60]. Like Na+ channels, many K+ channels have shown quite distinct and divergent gating properties depending on the types of auxiliary proteins. K+ channels are classified into several types, including voltage-gated channels (KV), Ca2+-activated K+ channels (KCa), inwardly rectifying channels (inward rectifier) and Na+-activated K+ channels. In this review, we focused on the roles of several KV subfamilies (Table 2) and Ca2+-activated K+ channels (Table 2) in intrinsic firing properties of the cerebellar PCs.
Considering the Hodgkin-Huxley model, ionic flow carried by Na+ and K+ determines the active and passive properties of neurons. Of interest, K+ conductance controls the resting membrane potential, membrane resistance, neuronal excitability, duration of AP and delay time to fire AP spike. Electrophysiological observations and immunohistochemistry data have shown that various types of K+ channels are expressed in cerebellar PC soma and dendrites [61,62,63,64,65,66,67,68]. Gähwiler and Llano [61] observed that outward
Differently from KV3 family, some other types of K+ channels show distinct gating properties, for instance, KV1 and 4 subtypes are activated at subthreshold voltage whereas KV3 family shows high voltage-activating and fast deactivating voltage dependency [76]. Storm [77] categorized the subthreshold-activated K+ channels into 2 types depending on their sensitivity for 4-AP concentration and on the gating properties, A-type and D-type KV channels, governed by KV1.4, KV4.X and KV1.1, 1.2, 1.6, respectively. D-type K+ channels, sensitive to dendrotoxin (DTX) and low concentration of 4-AP, are well known for determining spike output timing, latency and threshold for AP firing onset and firing frequency [14,77,78,79,80,81]. In the cerebellar PCs, large depolarization can induce Na+ - Ca2+ coupling in dendritic area leading to slowly depolarized potential (SDP), facilitating Na+ burst spike generation. Pharmacological inhibition of the D-type currents shortens the SDP and reduces latency to Ca2+ spikes [65], suggesting that the D-type channels are involved in the regulation of dendritic excitability. Application of DTX in cerebellar slices also increases spontaneous rhythmic activity through the enhancement of rebound firing, indicating that D-type
Previous studies have shown that A-type and D-type K+ channels play similar physiological roles. For example, the A-type
Instead of dendritic Na+ channels, cerebellar PC dendrites express Ca2+ channels with high density. Therefore, strong depolarization causes synchronization of the passively conducted Na+ spike and Ca2+ spike in dendrites to shape the appropriate spiking activity of PCs. The influx of dendritic Ca2+ can activate an ionic flow carried by K+, Ca2+-activated K+ channels. These types of channels are classified into two; small conductance and large conductance Ca2+-activated K+ channel (SK and BK channel, respectively) (Table 3). Both SK and BK channels are also implicated in controlling the spiking activity in PCs [88]. When the dendritic membrane is depolarized by synaptic inputs, local [Ca2+]i is elevated through P/Q type Ca2+ channels, leading to the activation of the SK channels [89]. The SK channels are expressed in PC somata as well as in dendrites. Interestingly, dendrosomatic electrical coupling is governed by SK2 channels in PC soma and dendrite [90]. In addition, somatic and dendritic SK channels show distinct roles in regulating PC activity. SK channels in soma set the maximal level of PC firing frequency, and on the other hand, dendritic SK channels determine the extent of dendritic regions contributing to the firing rates of PCs. The other types of Ca2+-activated K+ channel, BK channels are also involved in the spiking activity in PCs. As we described above, co-activity of KV3 and BK channels modulates dendritic Na+ - Ca2+ coupling burst elicited by CF inputs through suppression of Na+ channel inactivation [68]. Both SK and BK channels are activated by Ca2+ entering through P/Q type Ca2+ channels and regulate PC firing patterns. However, these channels differently contribute to firing behavior in PCs. SK channels have an impact on the setting intrinsic firing frequency [90] whereas BK channels are involved in the regulation of AP waveform and presumably climbing fiber responses [68,91]. Recent studies have shown that CF-evoked pause of spontaneous firing and generation of burst firing in PCs require BK channels coupled to Ca2+ channels [92,93]. Because SK and BK channels in PCs play a critical role in the regulation of firing behavior, dysfunction of these channels has been implicated in cerebellar disease such as ataxia. Mutations in P/Q type Ca2+ channels resulted in the decrease in the precision of PC pacemaking activity and perfusion of 1-ethyl-2-benzimidazolinone (EBIO), KCa activator improves the regularity of spontaneous firing and motor performance [94]. In addition, dysregulation of SK and/or BK channels has been reported in various genetic ataxia animal models [94,95,96,97], suggesting that modulation of SK and BK channels may be therapeutic targets for cerebella disease and motor dysfunction.
ACTIVITY-DEPENDENT PLASTICITY OF INTRINSIC EXCITABILITY THROUGH ION CHANNEL MODULATION
Many theories have implicated that the potentiation or depression of the synaptic transmission at the PF-PC synapses and non-synaptic intrinsic plasticity are required for cerebellar-dependent learning and memory. Intriguingly, the intrinsic plasticity requires an activity-dependent modulation of ion channels (Fig. 2) [13,14,15,98,99,100]. The ion channels in the cerebellar PCs are regulated by various factors such as an activation of metabotropic receptor or synaptic plasticity-related intracellular signaling [13,15,100,101]. When the chronic changes in network activity occur, the neuronal activity is homeostatically regulated in order to maintain the stability of network activity [102,103,104,105]. Prolonged application of tetrodotoxin (TTX) to organotypic cultures of cerebellar slices exhibits downregulation of the intrinsic excitability [100]. This homeostatic intrinsic plasticity is derived from an augmentation of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel current (
Recently, plasticity of the intrinsic excitability of the cerebellar PCs has been found to be accompanied by the synaptic plasticity [13,15,107]. In fact, PF-PC synaptic LTP derives the long-term potentiation of intrinsic excitability (LTP-IE). LTP-IE requires the downregulation of SK2 channels in dendritic area. The SK channel-dependent intrinsic plasticity may play a role in shaping the cerebellar PC output to adjust the impact of synaptic inputs within optimal ranges. The LTP-IE dampens the impact of PF inputs on the firing behavior of PCs, enabling effects of non-potentiated, weaker synaptic inputs on cellular output signal to minimize [13]. Interestingly, although local dendritic Ca2+ signaling is enhanced after formation of LTP-IE, prior induction of intrinsic plasticity prevents subsequent induction of synaptic LTP in PCs. Therefore, the role of intrinsic excitability in signal processing in the PCs is quite distinct from other types of neurons such as hippocampal pyramidal neurons or cortical neurons because enhanced Ca2+ signaling has been regarded as enlargements of synaptic inputs and increases in possibility of subsequent plasticity induction [108,109]. Considering that one important role of SK channels is setting the firing frequency in physiological limits, SK channel-mediated LTP-IE may ensure that excitatory drive stays within the physiological limits and prevent non-specific subsequent synaptic plasticity induction thereby stabilizing and maximizing the information processing after learning.
In addition to the intrinsic plasticity followed by induction of LTP, the activity-dependent downregulation of PF-PC synaptic function accompanies the intrinsic plasticity. Yang and Santamaria [107] described that the potentiation of excitability in PCs is found following an induction of PF-PC LTD through downregulation of HCN channel conductance. Many observations have shown that the associative eyeblink conditioning training exerts modification of synaptic strength (PF-PC LTD) as well as intrinsic properties of the cerebellar PCs (potentiation of excitability) [110,111]. Those described that the activity-dependent plasticity of intrinsic excitability may be homeostatically regulated which is similar to hippocampal intrinsic plasticity [18,19]. Alternatively, Shim et al. [15] demonstrated that the intrinsic excitability is found to be decreased following induction of synaptic LTD. This result is parallel with the previous observation in which population spiking activity is attenuated by synaptic LTD induction [112]. Those contradictory results may be derived from different experimental conditions: Yang and Santamaria [107] delivered PF stimuli with somatic depolarization instead of CF activation whereas Shim et al. [15] synaptically induced synaptic plasticity by delivering simultaneous and conjunctive stimulation of PF and CF within specific time-window. Behavioral training could induce neural plasticity through divergent pathways. Thus, the intrinsic plasticity following synaptic depression may be presumably modulated in various aspects including potentiation or depression of firing rates, responsiveness of synaptic inputs or patterns of spiking activity to achieve maximizing the information storage in the cerebellar circuits.
Several observations have shown that associative eyeblink conditioning accompanies the experience- and use-dependent plasticity in the cerebellar cortex [113,114,115,116,117]. The experience-dependent plasticity of dendritic membrane excitability can be maintained 1 month after conditioning. Recently, it was shown that the delayed eyeblink conditioning increases intrinsic excitability and changes in AP waveforms, presumably derived from SK channel downregulation [118]. There are several evidence supporting that the memory trace in PCs is not just an increase or a decrease in firing rates. In fact, PC activity reflects adaptively timed activity pattern with intrinsic cellular mechanisms rather than a temporal pattern of excitatory or inhibitory synaptic inputs [113,114,115]. Taken together, experience-dependent modulation of PC intrinsic excitability is another form of memory engram for the cerebellardependent motor learning.
Since Ito hypothesized that synaptic LTD between PF-PC synapses is the principal elements for the cerebellar-dependent motor learning [24], much of investigation has been extensively focused on the cellular mechanism of the modification of synaptic function to account memory storage in the cerebellum and motor control. Unlikely to synaptic plasticity or LTP-IE, the underlying mechanism for LTD-IE has less been elucidated. Previous reports described that the intrinsic plasticity and synaptic plasticity indeed share intracellular signaling including protein phosphatase and/or protein kinases (Fig. 2). Several signaling molecules are required to induce PF-PC LTD such as protein kinase C (PKC) and CaMKII. In the hippocampus, HCN channel activity is mediated by PKC signaling during mGluR-dependent plasticity induction, which results in intrinsic plasticity. However, mGluR-PKC signaling suppresses
Intrinsic plasticity plays a complementary role in integrating synaptic inputs and generating cellular output signal [15,18,19,105,123,124]. Synaptic-driven potentiation or depression of excitability show the same polarity with the corresponding direction of synaptic plasticity [13,15], indicating that the modifications in synaptic strength are synergistically reflected into the final net output of PCs following plasticity. When the synaptic weight is strengthened, the PC output signal is more potentiated, not compensated by intrinsic properties. In addition, cerebellar PC intrinsic plasticity occurs in the branch-dependent manner [98], indicating that synaptic inputs from specific-branches are potentiated by increased membrane excitability limited in conditioned dendritic branches. Otherwise, the plastic changes in synaptic transmission might be contaminated by global changes of excitability and less reflected into the neuronal firing output signal.
What is the physiological consequence of the bidirectional modulation of intrinsic excitability following plasticity induction or behavioral training? Vestibulo-ocular reflex (VOR) and optokinetic response (OKR) is a representative behavioral paradigm of cerebellar-dependent motor learning. The VOR gain, the ratio of vestibular stimuli to adaptive eye-movement, can be increased or decreased depending on the learning paradigm. Boyden and Raymond [37] reported that the VOR gain-up and -down learning are selectively engaged by the aspects of synaptic plasticity. There was a supporting evidence of this view in which injection of mGluR1 antagonist into the cerebellar flocculus, the core area for VOR learning in the cerebellum, suppresses gain-up learning whereas gain-down learning is not affected by either agonist and antagonist of mGluR1 [38]. In addition, an ultrastructural observation shows a reduction of surface AMPA receptor following the adaptive eye-movement learning, suggesting that the cerebellar LTD would occur during the motor learning [125]. Most recently, it was revealed that the adaptive eye-movement training is associated with modifications of synaptic transmission at the PF-PC synapses [6], indicating the prominent roles of bidirectional synaptic plasticity at PF-PC synapses in the motor learning.
In contrast to Ito's cerebellar LTD theory, many studies have shown that the PF-PC LTD may not be a sufficient cellular mechanism underlying motor learnings in spite of abundant studies describing the critical roles of PF-PC LTD in VOR learning [28,36,126,127,128,129]. Miles and Lisberger [130,131] proposed an alternative mechanism for motor learning, which suggests principal roles of VN neurons in the motor memory storage. There is, however, accumulating evidence showing that motor memory storage requires neuronal plasticity at multiple sites including neurons in the cerebellar cortex and VN [12,27]. Recently, a computational model for motor memory storage provided insights into the mechanism by which motor memory traces are seemingly transferred from cortical neurons (PC) to sub-cortical region (VN) [33,132]. Cumulative experimental data has shown that encoding the adaptive motor memory in the cerebellar cortex occurs within a few hours and the critical time window for memory transfer is approximately 2.5~4 hours after training [133,134]. Interestingly, Ito [129] recently suggested that an early adaption is dependent on the cerebellar cortical activity and the late phase of adaptation is accompanied by plasticity in the VN neurons. In parallel, the changes in the population spiking activity in VN neurons are manifested a day after training whereas there is no significant alteration of VN activity within an hour after training [135]. Until recently, this prediction for memory consolidation mechanism has not been investigated in a cellular and circuit level. Recent papers suggested that the intrinsic plasticity of the PCs might be the mechanism of the memory transfer from cerebellar cortex to sub-cortical areas [15,88]. Collectively, computational implications and experimental observations have both agreed to Ito's recent suggestion in which PF-PC synaptic plasticity may take part in the memory acquisition and plasticity in MF-VN synapses may be involved in long-term memory storage beyond two long-lasting conflicts for VOR memory: Marr-Albus-Ito's cerebellar LTD hypothesis vs. Miles and Lisberger' MF-VN synaptic plasticity theory.
Belmeguenai et al. [13] and Shim et al. [15] insisted that the intrinsic plasticity is modulated by synaptic activity pattern-dependent manner and this bidirectionality may function as an amplifier of the synaptic modification, enabling to transduce the finely tuned signal into the relay neurons such as neurons in DCN or VN. Given that the neural plasticity in the neurons in the cerebellar cortex and VN shows bidirectionality, the intrinsic plasticity would be also selectively engaged by certain forms of learning paradigm and the synergies with synaptic modulation may complete the scenario for the memory storage in the motor circuitry including cerebellum and VN.
THE FURTHER IMPLICATION OF INTRINSIC PLASTICITY IN THE MEMORY CIRCUITS
There are many unanswered questions to be solved in terms of the intrinsic plasticity and behavior. In the classical view of the intrinsic plasticity, changes in excitability have been considered to play pivotal roles in promoting subsequent induction of synaptic plasticity through the up- or downregulation of dendritic ion channels. Thus, the intrinsic plasticity of neurons has been thought as simply a supportive mechanism for activity-dependent modification of synaptic function although the information is conveyed by the AP spikes between neurons. However, some of the suggestions have described that activity-dependent modifications of ion channels can also undergo experience-dependent long-term plasticity beyond changes in synaptic weight [9,10,12,136,137,138,139]. Furthermore, cumulative evidence has shown that memory trace should move from one brain region to another brain region to consolidate the long-term memory [125,134,140,141,142,143,144]. Intrinsic plasticity may be one plausible mechanism of memory transfer via modulating the strength of connectivity between memory circuits.
Since the majority of researches have been performed to elucidate the mechanisms of cerebellar memory formation by using
In the neural circuitry for the cerebellar-dependent motor learning, it is unclear how the information is transferred to the subcortical area including DCN and VN neurons from cerebellar PCs is unclear. In the hippocampus, electrophysiological recording and optogenetic manipulation of neural circuits have revealed that the specific frequency of an oscillatory neural activity, called sharp wave ripple (SWR), involves in memory transfer from the hippocampus to cortex [145,146,147,148]. During learning, an interplay between synaptic and intrinsic plasticity increases the number of SWR replay events and thereby consolidating the long-term memory. Although the oscillatory activity of the cerebellum has been reported, detailed mechanisms and its physiological roles in formation of memory engram in the motor learning circuits have yet to be investigated. During VOR learning, spike discharge in the cerebellar PCs shows a sinusoidal pattern in response to vestibular head movement [149,150,151]. Interestingly, the phase of the oscillatory pattern of PC firing activity is altered after the training session, indicating that the response timing to sensory stimuli may be endowed with plastic changes. Therefore, the intrinsic plasticity of PCs may modify the patterns to integrate the synaptic inputs and to generate the spike discharge in response to sensory information.
Since memory is stored throughout the brain region, synergistic modulation of circuit dynamics might play critical roles. Various cutting-edge technologies enable to monitor the activity of large neuronal population and to manipulate the strength of neural circuits and corresponding behavioral outcomes. For example, recent studies using wide-field and high resolution in-vivo two-photon Ca2+ imaging approaches from behaving animals revealed the groundbreaking finding of the how the cerebellar granule cells process the sensory information [152,153]. These results from
Figures


Tables
| NaV1.6 (Resurgent Na+ channel) | ||
|---|---|---|
| Expression | Dendrite, Node of ranvier | [51] |
| Gating properties | Sensitivity for tetrodotoxin | [5355] |
| Evoked by a step repolarization to −30 mV | ||
| Maximum current at Vm=−30~−40 mV | ||
| V1/2 activation=−40 mV, rising time=5~6 ms | ||
| V1/2 inactivation=−62 mV (low Na+), −53 mV (high Na+), τdecay=20~30 ms | ||
| Impact on excitability | Reopening NaV when the membrane potential is repolarized to approximately −40 mV | [525455] |
| Shortens the refractory period between action potentials, high-frequency firing appears to be facilitated | ||
| Ablation | Reduced spontaneous firing rates | [545556575859] |
| Increased spike adaptation | ||
| Cerebellar ataxia & Dysfunction of motor coordination | ||
| Impairment of water maze and delayed eyeblink conditioning | ||
| KV1.4 & KV4 (A-type K+ channel) | ||
|---|---|---|
| Expression | Dendrite | [70] |
| Gating properties | Sensitivity for high concentration of 4-AP about 1~10 mM (insensitive for DTX) | [607785] |
| Fast-activating and inactivating channel | ||
| Activated at subthreshold voltage around −60 mV | ||
| V1/2 activation=−24.9 mV; V1/2 inactivation=−69.2 mV | ||
| τdeactivation at −70 mV : 3~4 ms | ||
| Impact on excitability | Acceleration of AP spike | |
| Firing frequency firing pattern (rhythmic Na-Ca spike burst) | ||
| Subthreshold variation of membrane properties | ||
| Impact on plasticity and learning | Eyeblink conditioning derives dendritic excitability underlying downregulation of A-type K+ channel | [116117] |
| KV1.1, KV1.2, KV1.6 (D-type K+ channel) | ||
|---|---|---|
| Expression | Dendrite | [70] |
| Gating properties | Sensitivity for low concentration of 4-AP about 0.2~1 mM and DTX (2.8~25 nM) | [6077] |
| Low-threshold and non-inactivating channel | ||
| Activated at −40~−50 mV | ||
| V1/2 activation=−20~−30 mV (KV1.2: −5~5 mV) | ||
| τdeactivation=14~23 ms | ||
| Impact on excitability | Spike frequency and adaption, dendritic excitability | [8283] |
| Amplitude and duration of rebound depolarization | ||
| Spontaneous bursts | ||
| KV3.3 & KV3.4 | ||
|---|---|---|
| Expression | Soma and Dendrite | [6970] |
| Gating properties | Sensitivity for TEA | [68] |
| Rapid activating at suprathreshold and rapidly inactivating channel | ||
| Peak amplitude at 30 mV from −70 mV | ||
| V1/2 activation=−23.0 mV, τdecay=0.66 ms | ||
| Impact on excitability | Repolarize the membrane potential and maintain repetitive firing | [6871] |
| Dendritic burst firing through Ca2+- Na+ coupling | ||
| Impact on plasticity and learning | Deletion of KV3.1/3.3 causes ataxic behavior | [73] |
| SK channel (SK2 subfamily) | ||
|---|---|---|
| Expression | Soma and Dendrite | [90] |
| Gating properties | Voltage-independent and Ca2+dependent channel | [6089] |
| Activated by Ca2+ influx through P/Q type Ca2+ channel | ||
| Sensitivity for apamin (63 pM) | ||
| Impact on excitability | Regulation of firing frequency | [13909598] |
| Shaping fast afterhyperpolarization (AHP) amplitude | ||
| Climbing fiber-induced spike pause duration | ||
| Activity-dependent modulation of climbing fiber-evoked EPSP amplitude and dendritic local Ca2+ transient | ||
| Impact on plasticity and learning | Activity-dependent downregulation of SK channel by eyeblink conditioning | [13118] |
| Inhibition of SK channel prevents LTP-IE induction | ||
| BK channel | ||
|---|---|---|
| Expression | Soma and Dendrite | [90] |
| Gating properties | Voltage- and Ca2+-dependent | [60] |
| V1/2 activation=50 mV at 4 μM [Ca2+] to −30 mV at 100 μM [Ca2+] | ||
| Impact on excitability | Generation of burst firing through cooperating with KV3 channels in dendrite | [68919293] |
| Climbing fiber-evoked spike pause and burst firing coupled to Ca2+ channel | ||
| Shaping medium or slow component of AHP | ||
| Impact on plasticity and learning | Dysfunction of SK and BK channels is related to cerebellar ataxia | [94959697] |
References
- Hebb DO. The organization of behavior: a neuropsychological theory. New York, NY: Wiley, 1949.
- Shigemoto R, Abe T, Nomura S, Nakanishi S, Hirano T. Antibodies inactivating mGluR1 metabotropic glutamate receptor block long-term depression in cultured Purkinje cells. Neuron 1994;12:1245-1255.
- Kashiwabuchi N, Ikeda K, Araki K, Hirano T, Shibuki K, Takayama C, Inoue Y, Kutsuwada T, Yagi T, Kang Y, Aizawa S, Mishina M. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR δ 2 mutant mice. Cell 1995;81:245-252.
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 1996;87:1327-1338.
- Shouval HZ, Bear MF, Cooper LN. A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proc Natl Acad Sci U S A 2002;99:10831-10836.
- Inoshita T, Hirano T. Occurrence of long-term depression in the cerebellar flocculus during adaptation of optokinetic response. eLife 2018;7:e36209.
- Nguyen-Vu TB, Zhao GQ, Lahiri S, Kimpo RR, Lee H, Ganguli S, Shatz CJ, Raymond JL. A saturation hypothesis to explain both enhanced and impaired learning with enhanced plasticity. eLife 2017;6:e20147.
- Gittis AH, du Lac S. Intrinsic and synaptic plasticity in the vestibular system. Curr Opin Neurobiol 2006;16:385-390.
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci 2003;4:885-900.
- Kim SJ, Linden DJ. Ubiquitous plasticity and memory storage. Neuron 2007;56:582-592.
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci 2004;7:126-135.
- Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci 2012;13:619-635.
- Belmeguenai A, Hosy E, Bengtsson F, Pedroarena CM, Piochon C, Teuling E, He Q, Ohtsuki G, De Jeu MT, Elgersma Y, De Zeeuw CI, Jörntell H, Hansel C. Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J Neurosci 2010;30:13630-13643.
- Hyun JH, Eom K, Lee KH, Ho WK, Lee SH. Activity-dependent downregulation of D-type K+ channel subunit Kv1.2 in rat hippocampal CA3 pyramidal neurons. J Physiol 2013;591:5525-5540.
- Shim HG, Jang DC, Lee J, Chung G, Lee S, Kim YG, Jeon DE, Kim SJ. Long-term depression of intrinsic excitability accompanied by synaptic depression in cerebellar Purkinje cells. J Neurosci 2017;37:5659-5669.
- Rancz EA, Häusser M. Dendritic spikes mediate negative synaptic gain control in cerebellar Purkinje cells. Proc Natl Acad Sci U S A 2010;107:22284-22289.
- Mittmann W, Häusser M. Linking synaptic plasticity and spike output at excitatory and inhibitory synapses onto cerebellar Purkinje cells. J Neurosci 2007;27:5559-5570.
- Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in
I h in hippocampal CA1 pyramidal neurons. J Neurosci 2007;27:13926-13937. - Fan Y, Fricker D, Brager DH, Chen X, Lu HC, Chitwood RA, Johnston D. Activity-dependent decrease of excitability in rat hippocampal neurons through increases in
I h. Nat Neurosci 2005;8:1542-1551. - Jung SC, Hoffman DA. Biphasic somatic A-type K channel downregulation mediates intrinsic plasticity in hippocampal CA1 pyramidal neurons. PLoS One 2009;4:e6549.
- Nelson AB, Faulstich M, Moghadam S, Onori K, Meredith A, du Lac S. BK channels are required for multisensory plasticity in the oculomotor system. Neuron 2017;93:211-220.
- Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin D, Thompson RF, Siegelbaum SA, Kandel ER, Morozov A. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell 2003;115:551-564.
- Vaidya SP, Johnston D. Temporal synchrony and gamma-to-theta power conversion in the dendrites of CA1 pyramidal neurons. Nat Neurosci 2013;16:1812-1820.
- Ito M. Cerebellar control of the vestibulo-ocular reflex--around the flocculus hypothesis. Annu Rev Neurosci 1982;5:275-296.
- Ito M. Long-term depression as a memory process in the cerebellum. Neurosci Res 1986;3:531-539.
- Ito M. Historical review of the significance of the cerebellum and the role of Purkinje cells in motor learning. Ann N Y Acad Sci 2002;978:273-288.
- Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci 2004;27:581-609.
- Ke MC, Guo CC, Raymond JL. Elimination of climbing fiber instructive signals during motor learning. Nat Neurosci 2009;12:1171-1179.
- Guo CC, Ke MC, Raymond JL. Cerebellar encoding of multiple candidate error cues in the service of motor learning. J Neurosci 2014;34:9880-9890.
- Kimpo RR, Rinaldi JM, Kim CK, Payne HL, Raymond JL. Gating of neural error signals during motor learning. eLife 2014;3:e02076.
- Streng ML, Popa LS, Ebner TJ. Climbing fibers predict movement kinematics and performance errors. J Neurophysiol 2017;118:1888-1902.
- Bloedel JR, Bracha V. On the cerebellum, cutaneomuscular reflexes, movement control and the elusive engrams of memory. Behav Brain Res 1995;68:1-44.
- Yamazaki T, Nagao S. A computational mechanism for unified gain and timing control in the cerebellum. PLoS One 2012;7:e33319.
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron 2005;48:647-659.
- Suvrathan A, Payne HL, Raymond JL. Timing rules for synaptic plasticity matched to behavioral function. Neuron 2016;92:959-967.
- Jörntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron 2006;52:227-238.
- Boyden ES, Katoh A, Pyle JL, Chatila TA, Tsien RW, Raymond JL. Selective engagement of plasticity mechanisms for motor memory storage. Neuron 2006;51:823-834.
- Titley HK, Heskin-Sweezie R, Broussard DM. The bidirectionality of motor learning in the vestibulo-ocular reflex is a function of cerebellar mGluR1 receptors. J Neurophysiol 2010;104:3657-3666.
- Hodgkin AL, Huxley AF. Resting and action potentials in single nerve fibres. J Physiol 1945;104:176-195.
- Hodgkin AL, Huxley AF, Katz B. Measurement of current-voltage relations in the membrane of the giant axon of
Loligo . J Physiol 1952;116:424-448. - Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 1952;117:500-544.
- Hodgkin AL, Huxley AF. Currents carried by sodium and potassium ions through the membrane of the giant axon of
Loligo . J Physiol 1952;116:449-472. - Stuart G, Häusser M. Initiation and spread of sodium action potentials in cerebellar Purkinje cells. Neuron 1994;13:703-712.
- Clark BA, Monsivais P, Branco T, London M, Häusser M. The site of action potential initiation in cerebellar Purkinje neurons. Nat Neurosci 2005;8:137-139.
- Brysch W, Creutzfeldt OD, Lüno K, Schlingensiepen R, Schlingensiepen KH. Regional and temporal expression of sodium channel messenger RNAs in the rat brain during development. Exp Brain Res 1991;86:562-567.
- Vega-Saenz de Miera EC, Rudy B, Sugimori M, Llinás R. Molecular characterization of the sodium channel subunits expressed in mammalian cerebellar Purkinje cells. Proc Natl Acad Sci U S A 1997;94:7059-7064.
- Schaller KL, Caldwell JH. Expression and distribution of voltage-gated sodium channels in the cerebellum. Cerebellum 2003;2:2-9.
- de Ruiter MM, De Zeeuw CI, Hansel C. Voltage-gated sodium channels in cerebellar Purkinje cells of mormyrid fish. J Neurophysiol 2006;96:378-390.
- Callaway JC, Ross WN. Spatial distribution of synaptically activated sodium concentration changes in cerebellar Purkinje neurons. J Neurophysiol 1997;77:145-152.
- Kay AR, Sugimori M, Llinás R. Kinetic and stochastic properties of a persistent sodium current in mature guinea pig cerebellar Purkinje cells. J Neurophysiol 1998;80:1167-1179.
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel NaV1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A 2000;97:5616-5620.
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci 1997;17:4517-4526.
- Afshari FS, Ptak K, Khaliq ZM, Grieco TM, Slater NT, Mc-Crimmon DR, Raman IM. Resurgent Na currents in four classes of neurons of the cerebellum. J Neurophysiol 2004;92:2831-2843.
- Khaliq ZM, Gouwens NW, Raman IM. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. J Neurosci 2003;23:4899-4912.
- Raman IM, Bean BP. Inactivation and recovery of sodium currents in cerebellar Purkinje neurons: evidence for two mechanisms. Biophys J 2001;80:729-737.
- Valkova C, Liebmann L, Krämer A, Hübner CA, Kaether C. The sorting receptor Rer1 controls Purkinje cell function via voltage gated sodium channels. Sci Rep 2017;7:41248.
- Yan H, Pablo JL, Wang C, Pitt GS. FGF14 modulates resurgent sodium current in mouse cerebellar Purkinje neurons. eLife 2014;3:e04193.
- Bosch MK, Carrasquillo Y, Ransdell JL, Kanakamedala A, Ornitz DM, Nerbonne JM. Intracellular FGF14 (iFGF14) is required for spontaneous and evoked firing in cerebellar Purkinje neurons and for motor coordination and balance. J Neurosci 2015;35:6752-6769.
- Woodruff-Pak DS, Green JT, Levin SI, Meisler MH. Inactivation of sodium channel
Scn8A (NaV1.6) in Purkinje neurons impairs learning in Morris water maze and delay but not trace eyeblink classical conditioning. Behav Neurosci 2006;120:229-240. - Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci 1999;868:233-285.
- Gähwiler BH, Llano I. Sodium and potassium conductances in somatic membranes of rat Purkinje cells from organotypic cerebellar cultures. J Physiol 1989;417:105-122.
- Gruol DL, Dionne VE, Yool AJ. Multiple voltage-sensitive K+ channels regulate dendritic excitability in cerebellar Purkinje neurons. Neurosci Lett 1989;97:97-102.
- Gruol DL, Jacquin T, Yool AJ. Single-channel K+ currents recorded from the somatic and dendritic regions of cerebellar Purkinje neurons in culture. J Neurosci 1991;11:1002-1015.
- Etzion Y, Grossman Y. Potassium currents modulation of calcium spike firing in dendrites of cerebellar Purkinje cells. Exp Brain Res 1998;122:283-294.
- Etzion Y, Grossman Y. Highly 4-aminopyridine sensitive delayed rectifier current modulates the excitability of guinea pig cerebellar Purkinje cells. Exp Brain Res 2001;139:419-425.
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci 1999;19:1663-1674.
- Martina M, Yao GL, Bean BP. Properties and functional role of voltage-dependent potassium channels in dendrites of rat cerebellar Purkinje neurons. J Neurosci 2003;23:5698-5707.
- McKay BE, Turner RW. Kv3 K+ channels enable burst output in rat cerebellar Purkinje cells. Eur J Neurosci 2004;20:729-739.
- Southan AP, Robertson B. Electrophysiological characterization of voltage-gated K+ currents in cerebellar basket and Purkinje cells: Kv1 and Kv3 channel subfamilies are present in basket cell nerve terminals. J Neurosci 2000;20:114-122.
- Sekirnjak C, Martone ME, Weiser M, Deerinck T, Bueno E, Rudy B, Ellisman M. Subcellular localization of the K+ channel subunit Kv3.1b in selected rat CNS neurons. Brain Res 1997;766:173-187.
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci 2001;24:517-526.
- Lien CC, Jonas P. Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. J Neurosci 2003;23:2058-2068.
- Matsukawa H, Wolf AM, Matsushita S, Joho RH, Knöpfel T. Motor dysfunction and altered synaptic transmission at the parallel fiber-Purkinje cell synapse in mice lacking potassium channels Kv3.1 and Kv3.3. J Neurosci 2003;23:7677-7684.
- Midtgaard J, Lasser-Ross N, Ross WN. Spatial distribution of Ca2+ influx in turtle Purkinje cell dendrites
in vitro : role of a transient outward current. J Neurophysiol 1993;70:2455-2469. - Llinás R, Sugimori M. Electrophysiological properties of
in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol 1980;305:197-213. - Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci 1999;868:233-285.
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature 1988;336:379-381.
- Harvey AL. Twenty years of dendrotoxins. Toxicon 2001;39:15-26.
- Dodson PD, Forsythe ID. Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends Neurosci 2004;27:210-217.
- Cudmore RH, Fronzaroli-Molinieres L, Giraud P, Debanne D. Spike-time precision and network synchrony are controlled by the homeostatic regulation of the D-type potassium current. J Neurosci 2010;30:12885-12895.
- Ovsepian SV, Steuber V, Le Berre M, O'Hara L, O'Leary VB, Dolly JO. A defined heteromeric KV1 channel stabilizes the intrinsic pacemaking and regulates the output of deep cerebellar nuclear neurons to thalamic targets. J Physiol 2013;591:1771-1791.
- Haghdoust H, Janahmadi M, Behzadi G. Physiological role of dendrotoxin-sensitive K+ channels in the rat cerebellar Purkinje neurons. Physiol Res 2007;56:807-813.
- Khavandgar S, Walter JT, Sageser K, Khodakhah K. Kv1 channels selectively prevent dendritic hyperexcitability in rat Purkinje cells. J Physiol 2005;569:545-557.
- Hounsgaard J, Midtgaard J. Intrinsic determinants of firing pattern in Purkinje cells of the turtle cerebellum
in vitro . J Physiol 1988;402:731-749. - Sacco T, Tempia F. A-type potassium currents active at subthreshold potentials in mouse cerebellar Purkinje cells. J Physiol 2002;543:505-520.
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 1997;387:869-875.
- Johnston D, Hoffman DA, Poolos NP. Potassium channels and dendritic function in hippocampal pyramidal neurons. Epilepsia 2000;41:1072-1073.
- Ryu C, Jang DC, Jung D, Kim YG, Shim HG, Ryu HH, Lee YS, Linden DJ, Worley PF, Kim SJ. STIM1 regulates somatic Ca2+ signals and intrinsic firing properties of cerebellar Purkinje neurons. J Neurosci 2017;37:8876-8894.
- Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci 2004;24:8818-8822.
- Womack MD, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar Purkinje neurons. J Neurosci 2003;23:2600-2607.
- Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol 2003;548:53-69.
- Jin XH, Wang HW, Zhang XY, Chu CP, Jin YZ, Cui SB, Qiu DL. Mechanisms of spontaneous climbing fiber discharge-evoked pauses and output modulation of cerebellar Purkinje cell in mice. Front Cell Neurosci 2017;11:247.
- Irie T, Trussell LO. Double-nanodomain coupling of calcium channels, ryanodine receptors, and BK channels controls the generation of burst firing. Neuron 2017;96:856-870.e4.
- Walter JT, Alviña K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci 2006;9:389-397.
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci U S A 2004;101:9474-9478.
- Dell'Orco JM, Wasserman AH, Chopra R, Ingram MA, Hu YS, Singh V, Wulff H, Opal P, Orr HT, Shakkottai VG. Neuronal atrophy early in degenerative ataxia is a compensatory mechanism to regulate membrane excitability. J Neurosci 2015;35:11292-11307.
- Egorova PA, Zakharova OA, Vlasova OL, Bezprozvanny IB.
In vivo analysis of cerebellar Purkinje cell activity in SCA2 transgenic mouse model. J Neurophysiol 2016;115:2840-2851. - Ohtsuki G, Piochon C, Adelman JP, Hansel C. SK2 channel modulation contributes to compartment-specific dendritic plasticity in cerebellar Purkinje cells. Neuron 2012;75:108-120.
- Hyun JH, Eom K, Lee KH, Bae JY, Bae YC, Kim MH, Kim S, Ho WK, Lee SH. Kv1.2 mediates heterosynaptic modulation of direct cortical synaptic inputs in CA3 pyramidal cells. J Physiol 2015;593:3617-3643.
- Shim HG, Jang SS, Jang DC, Jin Y, Chang W, Park JM, Kim SJ. mGlu1 receptor mediates homeostatic control of intrinsic excitability through
I h in cerebellar Purkinje cells. J Neurophysiol 2016;115:2446-2455. - Smith SL, Otis TS. Persistent changes in spontaneous firing of Purkinje neurons triggered by the nitric oxide signaling cascade. J Neurosci 2003;23:367-372.
- Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science 1994;264:974-977.
- Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci 1997;17:4527-4535.
- Cudmore RH, Turrigiano GG. Long-term potentiation of intrinsic excitability in LV visual cortical neurons. J Neurophysiol 2004;92:341-348.
- Nataraj K, Le Roux N, Nahmani M, Lefort S, Turrigiano G. Visual deprivation suppresses L5 pyramidal neuron excitability by preventing the induction of intrinsic plasticity. Neuron 2010;68:750-762.
- Rinaldi A, Defterali C, Mialot A, Garden DL, Beraneck M, Nolan MF. HCN1 channels in cerebellar Purkinje cells promote late stages of learning and constrain synaptic inhibition. J Physiol 2013;591:5691-5709.
- Yang Z, Santamaria F. Purkinje cell intrinsic excitability increases after synaptic long term depression. J Neurophysiol 2016;116:1208-1217.
- Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A 2002;99:8366-8371.
- Ramakers GM, Storm JF. A postsynaptic transient K+ current modulated by arachidonic acid regulates synaptic integration and threshold for LTP induction in hippocampal pyramidal cells. Proc Natl Acad Sci U S A 2002;99:10144-10149.
- Hauge SA, Tracy JA, Baudry M, Thompson RF. Selective changes in AMPA receptors in rabbit cerebellum following classical conditioning of the eyelid-nictitating membrane response. Brain Res 1998;803:9-18.
- Fiala JC, Grossberg S, Bullock D. Metabotropic glutamate receptor activation in cerebellar Purkinje cells as substrate for adaptive timing of the classically conditioned eye-blink response. J Neurosci 1996;16:3760-3774.
- Lev-Ram V, Mehta SB, Kleinfeld D, Tsien RY. Reversing cerebellar long-term depression. Proc Natl Acad Sci U S A 2003;100:15989-15993.
- Johansson F, Jirenhed DA, Rasmussen A, Zucca R, Hesslow G. Memory trace and timing mechanism localized to cerebellar Purkinje cells. Proc Natl Acad Sci U S A 2014;111:14930-14934.
- Halverson HE, Khilkevich A, Mauk MD. Relating cerebellar Purkinje cell activity to the timing and amplitude of conditioned eyelid responses. J Neurosci 2015;35:7813-7832.
- Jirenhed DA, Rasmussen A, Johansson F, Hesslow G. Learned response sequences in cerebellar Purkinje cells. Proc Natl Acad Sci U S A 2017;114:6127-6132.
- Schreurs BG, Tomsic D, Gusev PA, Alkon DL. Dendritic excitability microzones and occluded long-term depression after classical conditioning of the rabbit's nictitating membrane response. J Neurophysiol 1997;77:86-92.
- Schreurs BG, Gusev PA, Tomsic D, Alkon DL, Shi T. Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J Neurosci 1998;18:5498-5507.
- Titley HK, Watkins GV, Lin C, et al. Intrinsic excitability increase in cerebellar Purkinje cells following delay eyeblink conditioning in mice. bioRxiv 2018
- Smith MR, Nelson AB, Du Lac S. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. J Neurophysiol 2002;87:2031-2042.
- Nelson AB, Gittis AH, du Lac S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron 2005;46:623-631.
- McElvain LE, Bagnall MW, Sakatos A, du Lac S. Bidirectional plasticity gated by hyperpolarization controls the gain of postsynaptic firing responses at central vestibular nerve synapses. Neuron 2010;68:763-775.
- van Welie I, du Lac S. Bidirectional control of BK channel open probability by CAMKII and PKC in medial vestibular nucleus neurons. J Neurophysiol 2011;105:1651-1659.
- Mahon S, Charpier S. Bidirectional plasticity of intrinsic excitability controls sensory inputs efficiency in layer 5 barrel cortex neurons
in vivo . J Neurosci 2012;32:11377-11389. - Grasselli G, He Q, Wan V, Adelman JP, Ohtsuki G, Hansel C. Activity-dependent plasticity of spike pauses in cerebellar Purkinje cells. Cell Reports 2016;14:2546-2553.
- Wang W, Nakadate K, Masugi-Tokita M, Shutoh F, Aziz W, Tarusawa E, Lorincz A, Molnár E, Kesaf S, Li YQ, Fukazawa Y, Nagao S, Shigemoto R. Distinct cerebellar engrams in short-term and long-term motor learning. Proc Natl Acad Sci U S A 2014;111:E188-E193.
- Wulff P, Schonewille M, Renzi M, Viltono L, Sassoè-Pognetto M, Badura A, Gao Z, Hoebeek FE, van Dorp S, Wisden W, Farrant M, De Zeeuw CI. Synaptic inhibition of Purkinje cells mediates consolidation of vestibulo-cerebellar motor learning. Nat Neurosci 2009;12:1042-1049.
- Schonewille M, Belmeguenai A, Koekkoek SK, Houtman SH, Boele HJ, van Beugen BJ, Gao Z, Badura A, Ohtsuki G, Amerika WE, Hosy E, Hoebeek FE, Elgersma Y, Hansel C, De Zeeuw CI. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron 2010;67:618-628.
- Schonewille M, Gao Z, Boele HJ, Veloz MF, Amerika WE, Simek AA, De Jeu MT, Steinberg JP, Takamiya K, Hoebeek FE, Linden DJ, Huganir RL, De Zeeuw CI. Reevaluating the role of LTD in cerebellar motor learning. Neuron 2011;70:43-50.
- Ito M. Error detection and representation in the olivocerebellar system. Front Neural Circuits 2013;7:1.
- Miles FA, Lisberger SG. The “error” signals subserving adaptive gain control in the primate vestibulo-ocular reflex. Ann N Y Acad Sci 1981;374:513-525.
- Miles FA, Lisberger SG. Plasticity in the vestibuloocular reflex: a new hypothesis. Annu Rev Neurosci 1981;4:273-299.
- Clopath C, Badura A, De Zeeuw CI, Brunel N. A cerebellar learning model of vestibulo-ocular reflex adaptation in wild-type and mutant mice. J Neurosci 2014;34:7203-7215.
- Kassardjian CD, Tan YF, Chung JY, Heskin R, Peterson MJ, Broussard DM. The site of a motor memory shifts with consolidation. J Neurosci 2005;25:7979-7985.
- Okamoto T, Endo S, Shirao T, Nagao S. Role of cerebellar cortical protein synthesis in transfer of memory trace of cerebellum-dependent motor learning. J Neurosci 2011;31:8958-8966.
- Shutoh F, Ohki M, Kitazawa H, Itohara S, Nagao S. Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience 2006;139:767-777.
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci 2009;12:1438-1443.
- Park S, Kramer EE, Mercaldo V, Rashid AJ, Insel N, Frankland PW, Josselyn SA. Neuronal allocation to a hippocampal engram. Neuropsychopharmacology 2016;41:2987-2993.
- Lisman J, Cooper K, Sehgal M, Silva AJ. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat Neurosci 2018;21:309-314.
- Crestani AP, Krueger JN, Barragan EV, Nakazawa Y, Nemes SE, Quillfeldt JA, Gray JA, Wiltgen BJ. Metaplasticity contributes to memory formation in the hippocampus. Neuropsychopharmacology 2018
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci 2005;6:119-130.
- Restivo L, Vetere G, Bontempi B, Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J Neurosci 2009;29:8206-8214.
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci 2011;34:259-288.
- Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 2013;23:R764-R773.
- Nagao S, Honda T, Yamazaki T. Transfer of memory trace of cerebellum-dependent motor learning in human prism adaptation: a model study. Neural Netw 2013;47:72-80.
- Buzsáki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res 1998;:17-23.
- Carr MF, Jadhav SP, Frank LM. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci 2011;14:147-153.
- Poo MM, Pignatelli M, Ryan TJ, Tonegawa S, Bonhoeffer T, Martin KC, Rudenko A, Tsai LH, Tsien RW, Fishell G, Mullins C, Gonçalves JT, Shtrahman M, Johnston ST, Gage FH, Dan Y, Long J, Buzsáki G, Stevens C. What is memory? The present state of the engram. BMC Biol 2016;14:40.
- Tang W, Jadhav SP. Sharp-wave ripples as a signature of hippocampal-prefrontal reactivation for memory during sleep and waking states. Neurobiol Learn Mem 2018
- Mizukoshi K, Kobayashi H, Ohashi N, Watanabe Y. Quantitative analysis of the human visual vestibulo-ocular reflex in sinusoidal rotation. Acta Otolaryngol Suppl 1983;393:58-64.
- Fukushima K, Chin S, Fukushima J, Tanaka M. Simple-spike activity of floccular Purkinje cells responding to sinusoidal vertical rotation and optokinetic stimuli in alert cats. Neurosci Res 1996;24:275-289.
- Fukushima K, Buharin EV, Fukushima J. Responses of floccular Purkinje cells to sinusoidal vertical rotation and effects of muscimol infusion into the flocculus in alert cats. Neurosci Res 1993;17:297-305.
- Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L. Cerebellar granule cells encode the expectation of reward. Nature 2017;544:96-100.
- Giovannucci A, Badura A, Deverett B, Najafi F, Pereira TD, Gao Z, Ozden I, Kloth AD, Pnevmatikakis E, Paninski L, De Zeeuw CI, Medina JF, Wang SS. Cerebellar granule cells acquire a widespread predictive feedback signal during motor learning. Nat Neurosci 2017;20:727-734.
- Yamazaki T, Nagao S, Lennon W, Tanaka S. Modeling memory consolidation during posttraining periods in cerebellovestibular learning. Proc Natl Acad Sci U S A 2015;112:3541-3546.
- Gao Z, Proietti-Onori M, Lin Z, Ten Brinke MM, Boele HJ, Potters JW, Ruigrok TJ, Hoebeek FE, De Zeeuw CI. Excitatory cerebellar nucleocortical circuit provides internal amplification during associative conditioning. Neuron 2016;89:645-657.



