Berries and other natural products in pancreatic cancer chemoprevention in human clinical trials
Abstract
Pancreatic ductal adenocarcinoma (PDAC) was the 12th and 11th most common cancer in men and women worldwide in 2012, with the highest incidence in North America and Europe and the lowest in Africa and Asia. Due to the lack of efficient early diagnosis and rapid disease progression, PDAC patients have a 5-year survival rate of just 5%. Epidemiological studies suggest that smoking, obesity, type II diabetes, and pancreatitis are common risk factors for PDAC development. By contrast, high intake of fresh fruit, vegetables, and nuts rich in phytochemicals could reduce PDAC risk. This review summarizes the human clinical studies that have used berries or other natural products for chemoprevention of PDAC. Developing chemopreventive agents against PDAC would be tremendously valuable for the high-risk population and patients with premalignant lesions. Although some clinical trials of these agents have been completed, most are in early phases, and the results are not promising, which may be due to administration of the natural products at advanced stages of PDAC. Thus, further mechanistic studies using genetic animal models that recapitulate the tumor microenvironment and immunology of human PDAC would be informative for selecting an effective window for intervention with berries or other natural compounds.
1Introduction
Pancreatic cancer was the 12th most common cancer in men and the 11th in women worldwide in 2012 [1, 2]. The highest incidence was in North America and, especially, in Europe [2], which had one third of estimated new cases in 2012 [2]. The lowest incidence was in East Asia and Africa [2]. Because of its extremely high mortality rate, pancreatic cancer was the 7th leading cause of cancer-related deaths worldwide in 2012 [2]. In the United States, an estimated 53,670 new cases of pancreatic cancer and 43,090 pancreatic cancer-related mortalities will occur in 2017, making pancreatic cancer the 4th leading cause of cancer mortality in the U.S. [3]. More importantly, pancreatic cancer is projected to surpass breast, prostate, and colorectal cancer to become the 2nd leading cause of cancer-related death in the U.S. by 2030 [4]. Similarly, it is projected to be the 5th most common cancer and the 2nd leading cause of cancer mortality in Germany by 2030 [5]. Due to the lack of efficient early diagnosis, as well as aggressive disease progression and metastasis, pancreatic cancer patients have a 5-year survival rate of about 5% in all races, which is significantly lower than any other type of cancer [2, 3]. Although many unmodifiable risk factors could contribute to the development of pancreatic cancer, such as aging, race, family history, and genetic syndromes, many modifiable risk factors have been reported to strongly associate with disease incidence, including smoking, type II diabetes, obesity, inadequate physical exercise, and chronic pancreatitis [6, 7].
Most pancreatic cancer patients present with symptoms of weakness, loss of appetite, nausea, vomiting, jaundice, pain in the upper abdomen, and weight loss. Although several tumor biomarkers [8], especially carbohydrate antigen 19-9 (CA-19.9) [9], appear to have promising prognostic and predictive features, no biomarkers have been established for detecting early lesions. In most pancreatic cancer cases, the cancer has already metastasized to other organs by the time symptoms become noticeable, and surgical intervention may no longer be a viable option [10]. In 1997, gemcitabine became the first standard chemotherapeutic drug for metastatic pancreatic cancer because it produced a significant but moderate median overall survival (OS) benefit over 5-fluorouracil (5-FU) [11]. More combination regimens based on gemcitabine have been investigated, but only the combination of albumin-bound paclitaxel (nab-paclitaxel) and gemcitabine significantly improved OS (8.5 vs. 6.7 months, p < 0.001) and progress-free survival (PFS) (5.5 vs. 3.7 months, p < 0.001) compared to gemcitabine alone [12]. The other combinations with gemcitabine failed to generate meaningful improvements in OS. However, a gemcitabine-free combination, FOLFIRINOX (folinic acid, fluorouracil, irinotecan, and oxaliplatin) significantly improved OS benefit (11.1 vs. 6.8 months, p < 0.001) and PFS benefit (6.4 vs. 3.3 months, p < 0.001) compared to gemcitabine alone [13]. Unfortunately, both the nab-paclitaxel-gemcitabine and FOLFIRINOX regimens led to substantial toxicity, such as peripheral neuropathy and myelosuppression.
Almost half of pancreatic cancer patients reach out for a second-line therapy due to disease progression. The combinations of 5-FU with oxaliplatin or liposomal irinotecan are currently recommended for patients who fail gemcitabine-based first-line treatment/s [14–16], while a gemcitabine-based therapy is recommended as the second-line option for patients who received FLOFIRINOX as the first-line therapy. However, for patients who have progressed beyond two lines, no standard of care is available. Enrollment in clinical trials of novel treatments is advocated for these patients [17].
Due to the late stage of detection, cancer-induced cachexia is a large comorbidity in pancreatic cancer, as it not only significantly impacts patients’ overall quality of life but also decreases survival rates. Accordingly, there is an urgent need to develop and establish a reliable screening and early detection method, as well as to expand our understanding of the disease so we can find new approaches to preventing and treating pancreatic cancer by administering one or more combinations of natural and/or synthetic agents. In recent years, natural dietary compounds have gained increasing attention as adjuvant therapy due to their relative low toxicity and synergistic effects with current chemotherapeutic agents. In this review, we focus on the use of berries and other natural compounds for chemoprevention of pancreatic cancer, particularly in human clinical trials. For comprehensive summaries of pancreatic cancer prevention using natural products in cell culture models and animals, readers are referred to other excellent reviews [18, 19]. Interventional clinical trials of human pancreatic cancer using natural products are listed in Table 1.
Table 1
List of interventional clinical trials with pancreatic cancer patients using natural products
| Agent and Trial No. | Trial Status | Phase | No. of | Disease Stage | Location/Site | Sources of |
| Patients | nature products | |||||
| Curcumin | ||||||
| NCT00094445 (Ref 41) | Completed | II | 50 | Advanced, metastatic, or recurrent | M.D. Anderson Cancer Center | Sabinsa Corporation |
| NCT00192842 | Completed | II | 17 | Locally advanced or metastatic | Rambam Health Care Campus | – |
| NCT00486460 | Unknown | III | – | – | Tel-Aviv Sourasky Medical Center | – |
| Vitamin C (ascorbate) | ||||||
| NCT01049880 (Ref 79) | Completed | I | 15 | Metastatic | University of Iowa, Holden Comprehensive Cancer Center | Ascorbic Acid Injection USP, Bioniche Pharma |
| NCT02896907 | Recruiting | I | 8 | Locally advanced unresectable or Recurrent | Sidney Kimmel Cancer Center, Thomas Jefferson University | – |
| NCT00954525 (Ref 80) | Completed | I | 14 | Metastatic | Thomas Jefferson University | Ascorbic Acid Injection USP, Bioniche Pharma |
| NCT01905150 | Recruiting | II | 30 | Metastatic, locally advanced unresectable, or locally recurrent | Bruckner Oncology, Bronx, New York | – |
| Vitamin D | ||||||
| NCT02754726 | Recruiting | II | 10 | Metastatic | HonorHealth Research Institute | Paricalcitol |
| Vitamin E δ-tocotrienol | ||||||
| NCT00985777 (Ref 84) | Completed | I | 26 | Resectable | H. Lee Moffitt Cancer Center and Research Institute | Pure vitamin E δ-Tocotrienol |
| Fish oil | ||||||
| NCT01019382 (Ref 85) | Completed | II | 50 | Unresectable | University Hospitals, Leicester | Lipidem, B. Braun Melsungen AG |
| NCT01256034 (Ref 86) | Completed | IV | 50 | Resectable | Chiba University, Japan | Oral Impact®, Nestlé |
| NCT01789073 (Ref 87) | Completed | Not available | 35 | Resectable | University of Copenhagen | Oral Impact®, Nestlé |
| Fish oil + vitamin E | ||||||
| NCT02681601 | Not yet Recruiting | Not available | 60 | Unresectable | Jonsson Comprehensive Cancer Center, The University of California Los Angeles | OmegaRich, NutraWell nutrition powder, New Health Enterprises |
| Genistein | ||||||
| NCT00882765 | Withdrawn prior to enrollment | II | – | – | Jonsson Comprehensive Cancer Center, The University of California Los Angeles | – |
| Broccoli Sprout | ||||||
| NCT01879878 | Unknown | Not available | 40 | Advanced, unresectable | Heidelberg University, Germany | Broccoli sprout grain |
| L-CARnitine | ||||||
| NCT01330823 | Suspended | III | 72 | Advanced | University Medicine Greifswald | – |
| Dietary supplements without | ||||||
| defined ingredients | ||||||
| NCT01947166 | Completed | Not available | 38 | Resectable | Duke University | Nestle Impact Advanced Recovery nutritional supplement |
| NCT02745197 | Not yet Recruiting | II | 88 | Resectable | University of Alberta | – |
| NCT02626195 | Recruiting | II | 50 | Resectable | National Cancer Center, Korea | – |
| NCT02940067 | Recruiting | Not available | 40 | Resectable | University of Surrey | MEDiterranean diet |
| NCT00003851 | Terminated | 90 | Unresectable primary or metastatic | Columbia University | Gonzalez Regimen | |
| NCT00049608 | Terminated | I | 51 | Metastatic, recurrent, or unresectable locally advanced | National Cancer Institute (NCI) | Mistletoe extract |
| NCT01838109 | Completed | Not available | 174 | Resectable | Seoul National University Hospital | – |
| NCT01642875 | Recruiting | IV | 96 | Resectable Primary periampullary tumor | Medical University of Warsaw | – |
| NCT02670265 | Completed | Not available | 100 | Biliopancreatic Mass Lesions | University Medicine Greifswald | Olimel peri 2.5% ®, Baxter Germany GmbH Medication Delivery |
| NCT01419483 | Suspended | I | 5 | IIA, IIB, or III | University of Iowa | Ketogenic diet |
| NCT00919659 | Completed | II | 32 | With progressive cancer cachexia | CONKO-Studiengruppe | – |
| NCT02336087 | Recruiting | I | 21 | Unresectable | City of Hope Medical Center; NCI | Standardized Dietary Supplement |
2Materials and methods
We performed a PubMed and ClinicalTrials.gov search for publications from 1985 through 2017, using the following key words: pancreatic cancer, dietary supplement, berries, nutrition, diet, polyphenols, or human clinical trials.
Articles and clinical trials that described and compared the impact of natural products on pancreatic cancer were first screened according to abstracts and titles. Then selected articles were assessed for eligibility as full-text articles. No language restrictions were applied. Reference lists from studies selected by the electronic search were manually searched to identify further relevant reports. Reference lists from all available review articles and primary studies were also considered. The quality and level of strength of the results were also considered.
3Berries
In botanical terminology, a berry is a simple fruit that is indehiscent (doesn’t split open to release its seeds when ripe), has few-to-many seeds, and is derived from a single, simple, or compound ovary [20]. Thus, berries include many commonly consumed fruits and vegetables, such as strawberries, blueberries, blackberries, red raspberries, black raspberries, cranberries, grape, kiwi, banana, tomatoes, eggplant, cucumber, watermelon, etc., as well as many uncommon types, such as gooseberries, Goji berries, elderberries, noni (Morinda citrifolia), and acai (Euterpe oleracea Mart), etc. When we used the names of these berries mentioned above and “pancreatic cancer” as keywords to search publications, we identified one study that used blueberries and five other studies that used grape. They were limited to in vitro and in vivo studies. Although no berry studies on humans are available, these preclinical studies suggest that berries show promise for preventing pancreatic cancer in humans.
3.1Black raspberries
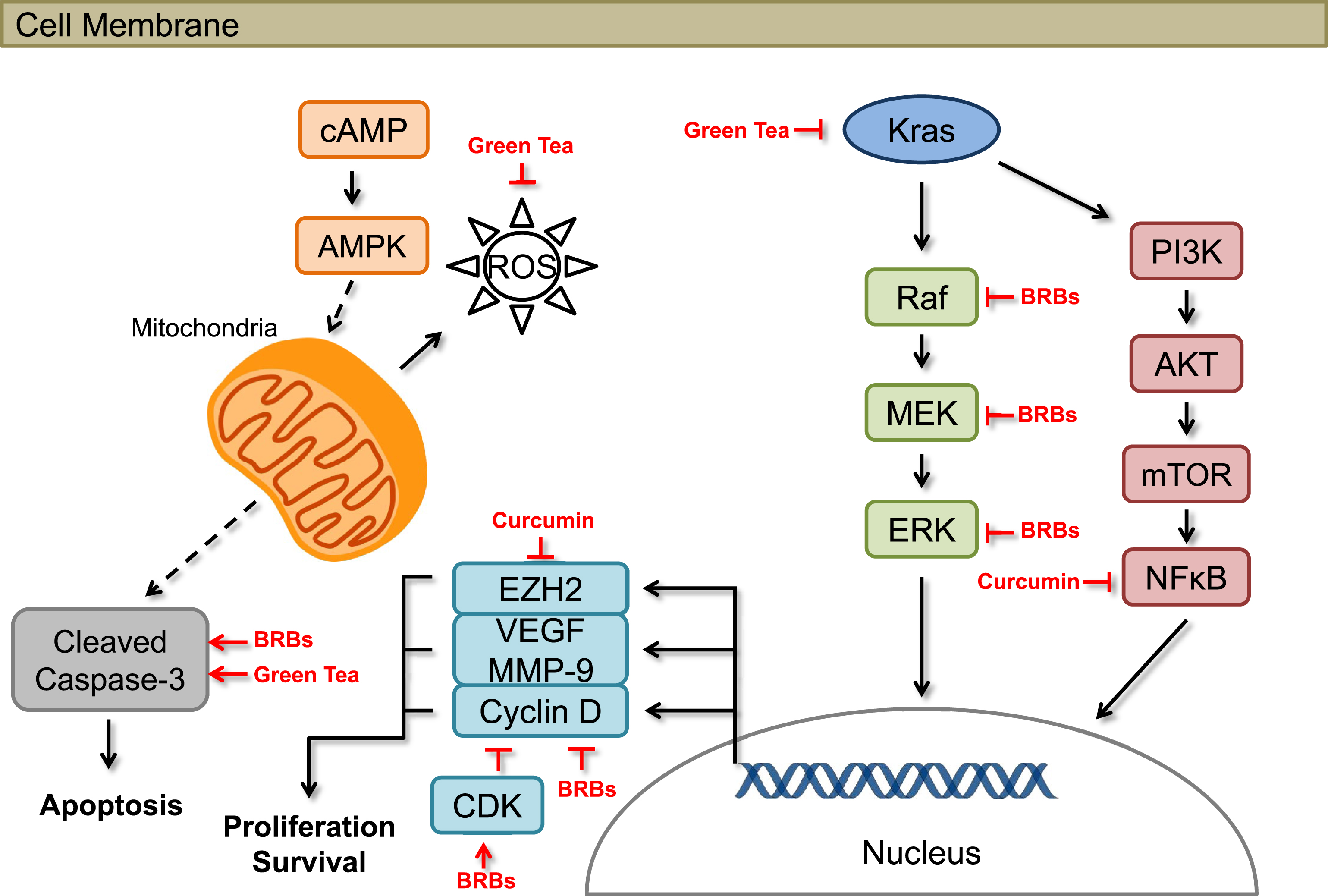
Black raspberries (BRBs) have been shown to have chemopreventive effects against colorectal cancer in human patients [21–23] and mouse models [24–26]. Our laborabory focuses on the potential efficacy of BRBs against pancreatic cancer. In one of our most recent studies, we investigated the potential effects of BRBs against pancreatic cancer in mice [27, 28]. Four-week old KrasLSL . G12D/+-Trp53LSL . R172H/+-Pdx-1-Cre (KPC) mice were fed either a control diet (AIN-76A) or the control diet supplemented with 5% BRBs. The KPC mice, which carry Kras and Trp53 mutations in the pancreas, spontaneously develop pancreatic cancer that molecularly and pathologically recapitulates human pancreatic cancer [29–31]. Kaplan-Meier survival analysis showed that 5% BRBs significantly prolonged the survival of the KPC mice, with a median survival of 189 days compared with 154 days for mice fed the control diet. Molecular studies further suggested that BRBs suppressed Raf/mitogen-activated protein kinase kinase (MAPKK, MEK)/mitogen-activated protein kinase (MAPK, ERK)/signal transducer and activator of transcription 3 (STAT3) pathways, which are all downstream targets of Kras activation. They also inhibited cancer cell proliferation in pancreatic tumor tissues (Fig. 1). Furthermore, 6 weeks of 5% BRB treatment significantly reduced tumor size in an immune-deficient mouse model lacking functional T and B cells (NOD.SCID mice orthotopically injected with Panc-1 cells) [32]. Additionally, orthotopic tumors in BRB-treated NOD.SCID mice had a higher rate of apoptosis than tumors from control diet-fed mice (Fig. 1). These results support the hypothesis that BRBs might have clinical potential for delaying pancreatic cancer progression through the suppression of cancer cell proliferation and/or promotion of cellular apoptosis. In addition, we investigated BRB’s ability to modulate pancreatic cancer immune surveillance. BRBs decreased tumor-infiltrated myeloid-derived suppressing cells (MDSC) and increased cytolytic natural killer (NK) cells in both KPC mice and NOD.SCID mice. BRBs also increased tumor-infiltrated CD8+ T cells in KPC mice. Expression of IL-12, a cytokine that activates both NK cells and CD8+ T cells, was increased by BRBs (manuscript under revision). These results suggest that BRBs have the potential to modulate cancer immune surveillance by reversing the immunosuppressive microenvironment and enhancing anti-tumorigenic NK cells and CD8+ T cells, thus prolonging the survival of mice bearing pancreatic tumors.
Fig.1
Effects of berries and other natural products on cellular pathways associated with pancreatic cancer in preclinical models.
3.2Blueberries
Pterostilbene (10–100μM), a stilbenoid derived from blueberries, was shown to inhibit cell proliferation and/or induce cell death, cell cycle arrest, mitochondrial membrane depolarization, and activation of effector signaling cascades in two pancreatic cancer cell lines (MIA PaCa-2 and Panc-1) [33].
3.3Grape
Grape seed procyanidin extract (GSPE) was shown to inhibit cell proliferation and induce apoptosis in pancreatic cancer cell lines (MIA PaCa-2, Panc-1, AsPC-1, and BxPC-3) [34–37]. It also inhibited the growth of MIA PaCa-2 pancreatic tumors in xenograft mice [35]. Gallic acid was identified as a major single active constituent of GSPE [34]. In addition, resveratrol, a phenolic compound found in grape skin, was shown to inhibit cell proliferation in human pancreatic cancer cell lines (CD18 and S2-013) [38].
4Other natural products
Many other natural products were evaluated, or are under investigation in human clinical trials, such as curcumin, green tea, vitamins, and fish oil (Table 1). Results from some early-phase trials, particularly vitamin C and vitamin D, may provide novel insight for combination therapies.
4.1Curcumin
Preclinical studies have shown that curcumin, which is derived from turmeric (Curcuma longa), has a wide range of effects, such as anti-inflammatory, pro-apoptotic, anti-proliferative, and anti-angiogenic activities (Fig. 1) [39–42]. Moreover, several human clinical trials have shown that curcumin is a safe treatment for pancreatic cancer patients. In a phase I/II study conducted at Kyoto University Hospital, oral curcumin was shown to be both safe and tolerable for gemcitabine-resistant pancreatic cancer patients [43]. Curcuminoids in this clinical trial were courteously provided by the Sabinsa Corporation (Piscataway, NJ) in microbead form [43]. Twenty-one patients with histologically or radiologically confirmed adenocarcinoma in the pancreas who showed disease progression during gemcitabine-based chemotherapy were included in that study. The patients received 8 g of oral curcumin daily in combination with gemcitabine-based chemotherapy. No patients withdrew from the study due to intolerability of curcumin, and the toxicity profile was comparable with that observed in pancreatic cancer patients treated with gemcitabine therapy alone. These findings support the possibility of supplementing gemcitabine-based chemotherapy with curcumin treatment. Encouragingly, several patients reported improvements in cancer- and chemotherapy-related symptoms such as fatigue, pain, and constipation after taking curcumin [43]. However, this study did not include a quality-of-life score among its pre-specified endpoints.
Another phase II trial investigated the clinical effects of curcumin treatment for patients with histologically confirmed pancreatic adenocarcinoma [44]. Curcumin was also obtained from Sabinsa Corporation as 1 g caplets [44]. Twenty-five patients were enrolled, with 21 evaluable for response to curcumin treatment. The patients received 8 g of oral curcumin daily until disease progression, and the disease was restaged every 2 months. Plasma curcumin levels remained relatively constant, ranging from 22 to 41 ng/mL [44]. Two patients showed clinical biological activities: the first had ongoing stable disease for more than 18 months, and the other had a brief (one-month) but dramatic period of tumor regression [44]. Furthermore, no toxicities due to curcumin were observed, and plasma curcumin levels were comparable with those in other studies [43].
Another clinical trial evaluated the activity and feasibility of gemcitabine in combination with curcumin in patients with advanced pancreatic cancer [45]. Seventeen patients received 8 g of oral curcumin (Sabinsa Corporation) daily combined with intravenous injection of gemcitabine (1,000 mg/m2) weekly for 3 weeks, followed by a 1-week rest. Five patients (29%) discontinued curcumin after a few days to 2 weeks due to intractable abdominal fullness or pain, and the dose of curcumin was reduced to 4 g/day because of abdominal complaints in 2 other patients. One of 11 evaluable patients had a partial response, 4 had stable disease, while 6 had tumor progression. Time to tumor progression ranged from 1–12 months, with a median of 2.5 months, and overall survival was between 1 and 24 months, with a median of 5 months [45]. Low compliance for curcumin at a dose of 8 g/day, when taken together with systemic gemcitabine, may prevent the use of the high doses of oral curcumin needed to achieve a systemic effect. Further studies should evaluate whether other formulations of curcumin could enhance the effects of chemotherapy.
In a recently published retrospective case-control study, the effects of curcumin on the body composition of 66 patients with advanced pancreatic cancer (22 patients on the treatment arm and 44 patients on the control arm) were evaluated [46]. The pancreatic cancer patients who were treated with curcumin lost significantly more subcutaneous fat and muscle than the untreated patients [46]. In the same study, sarcopenic patients treated with curcumin (n = 15) had a median survival of 169 (115–223) days compared to a median survival of 299 (229–369) days for the untreated sarcopenic patients (p = 0.024). No survival difference was found among the non-sarcopenic patients [46]. Therefore, the muscle loss associated with curcumin treatment should be taken into consideration when combining curcumin with other chemotherapies.
One difficulty with curcumin treatment is low bioavailability even when the supplement is taken in large doses [47]. The absorption and elimination half-lives of orally administered curcumin (2 g/kg) in rats were reported to be 0.31±0.07 and 1.00±0.5 h, respectively. A 2 g dose of curcumin resulted in undetectable serum curcumin levels in human patients [48]. In the two previously mentioned clinical trials with a dose of 8 g of curcumin, plasma curcumin levels ranged from 22–41 ng/mL and 29–412 ng/mL [43, 44], suggesting that individuals very widely. As plasma curcumin level is not a perfect representation of the supplement’s bioavailability in the pancreas, uptake of curcumin by the pancreas needs to be measured in a more direct manner before strong conclusions can be reached about bioavailability during curcumin treatment. For example, it can be measured in surgically removed pancreas in pre-surgical window-of-opportunity trials. Some studies have used curcumin analogues to increase bioavailability and treatment effectiveness. In the pancreatic cancer cell line PK-1, the curcumin analog GO-Y030 induced cell death to a similar extent to curcumin itself, but at a 10-fold lower concentration [49]. However, the bioavailability and safety of GO-Y030 in human pancreatic cancer patients has yet to be determined. Another promising curcumin analogue is difluorinated-curcumin (CDF), which was developed to enhance bioavailability specifically in pancreatic tissues. CDF accumulated in the pancreas of mice at a 10-times higher level than curcumin [50]. Furthermore, when CDF was conjugated with β-cyclodextrin, levels of the complex were 10 times higher in the pancreas than in serum [51]. The mechanism by which β-cyclodextrin increases bioavailability might be attributed to its hydrophobic core and hydrophilic outer layer, which allows for enhanced solubility of hydrophobic compounds such as curcumin and its analogues. These results are encouraging, as the bioavailability of curcumin is one of the key limitations to its effectiveness as a chemotherapeutic agent against pancreatic cancer. Furthermore, the toxicity and bioavailability of curcumin analogues need to be investigated to determine if they are safe and should be used in future clinical trials.
The efficacy of curcumin will also need to be investigated in a genetic model of pancreatic cancer. Particularly with respect to pancreatic cancer, tumor xenograft models have been largely disappointing in their ability to predict the therapeutic efficacy of treatments in humans. For example, curcumin was very effective in orthotopic mouse models [52, 53], but the results of human clinical trials with curcumin were not consistent with the orthotopic mouse experiments [44, 54]. Therefore, it would be useful to investigate the efficacy of curcumin and its analogues in a genetic model of pancreatic cancer, such as KrasLSL . G12D/+-Pdx-1-Cre (KC) mice, which carry the Kras mutation only in the pancreas, or KPC mouse models, which recapitulate the complex tumor microenvironment in human pancreatic cancer [55].
4.2Green tea polyphenols
Green tea made from the plant Camellia sinensis contains large amounts of polyphenols, specifically catechins such as (–)-Epigallocatechin-3-gallate (EGCG). These polyphenols have strong chemopreventive effects such as inhibiting the growth and invasion of pancreatic cancer cells and inducing apoptosis [56–63]. They also are strong antioxidants [64–70] both in vivo and in vitro (Fig. 1). In addition, the combination of EGCG and pterostilbene from blueberries had additive, anti-proliferative effects in vitro; it also altered apoptotic mechanisms in pancreatic cell lines (MIA PaCa-2 and Panc-1) [71].
Based on these promising results, green tea extracts were used in a double-blind, randomized, clinical trial as part of an oral antioxidant before surgery [72]. Thirty-six pancreatic cancer patients undergoing pancreaticoduodenectomy were randomized to receive either the pre-conditioning oral nutritional supplement (Fresenius Kabi, Bad Homburg, Germany) that contained the green tea extract or a placebo. The nutritional supplement or placebo was given twice a day before surgery as well as 3 hours before the surgery. On postoperative days 1, 3, and 7, total endogenous antioxidant capacity and plasma levels of several vitamins and proteins were measured along with markers for oxidative stress and systemic inflammatory markers. On postoperative day 1, plasma levels of vitamin C, selenium, and zinc were higher in the nutritional supplement group than in the placebo group. Furthermore, total endogenous antioxidant capacity was improved at all 3 time points in the nutritional supplement group. However, there was no difference in levels of oxidative stress and systemic inflammation markers between the two groups. One limitation of this study was that plasma levels provide only an approximation of endogenous antioxidant activity in the pancreas. Assessing antioxidant levels in surgical tissues would provide direct information on the effect of the nutritional supplement. Another possible limitation of the study was that the nutritional supplement was given only before surgery and not during the time when increased antioxidant activity might be most beneficial. It is likely that the positive effects from the nutritional supplement treatment seen on postoperative day 1 could have continued if nutritional supplementation had continued after the surgery. Further studies need to determine both the optimal dosage and length of nutritional supplementation that would produce the greatest anti-oxidative effect.
In recent years, several epidemiological studies have examined the effect of green tea intake on the risk of developing pancreatic cancer [73–77]. These studies have been largely inconclusive, as studies in China indicated a reduction in pancreatic cancer risk associated with green tea intake [73, 76], while others in Japan found no association [74, 75]. One major drawback to these epidemiological studies is that all the results were self-reported and therefore subject to bias. Also, measurements of green tea intake were also inconsistent between studies. For example, one Japanese study focused on the frequency of green tea intake (1–2 days/week; 3–4 days/week; etc.), while another used the total amount of green tea (grams of dry tea leaves) [73, 75]. Furthermore, all of these studies were conducted exclusively in China or Japan, and any results might not translate well to people in other areas. For example, one Japanese study reported that 79.2% of men and 76.6% of women said they consumed green tea every day [74]. It would be interesting to investigate the effects of green tea consumption in cultures where the intake of green tea is not as widespread. Thus, further laboratory and epidemiological research, especially involving a wider range of populations, is needed to validate the association between green tea consumption and pancreatic cancer risk.
4.3Vitamins
4.3.1Vitamin C (ascorbate)
Pharmacologic ascorbate can be cytotoxic because it can be oxidized to H2O2. Pancreatic cancer cells are sensitive to H2O2 generated by ascorbate, they would be expected to become sensitized to agents, such as ionizing radiation, that increase oxidative damage [78]. One study demonstrated that pharmacologic ascorbate enhanced the cytotoxic effects of ionizing radiation as seen by decreased cell viability and clonogenic survival in the pancreatic cancer cell lines examined but not in normal pancreatic ductal epithelial cells [78]. Radio-sensitization induced by ascorbate was associated with increased oxidative stress-induced DNA damage, which was reversed by catalase. In mice with established heterotopic and orthotopic pancreatic tumor xenografts, pharmacologic ascorbate combined with ionizing radiation decreased tumor growth and increased survival without damaging the gastrointestinal tract or increasing systemic oxidative stress.
A phase I clinical trial (PACMAN trial) was performed to establish the safety and tolerability of pharmacological ascorbate combined with gemcitabine in patients with biopsy-proven stage IV pancreatic adenocarcinoma [79]. Nine subjects were given intravenous ascorbate (15–125 g twice-weekly) (Bioniche Pharma USA, Lake Forest, IL), using Simon’s accelerated titration design to reach a targeted post-infusion plasma level of ≥350 mg/dL (≥20 mM). Meanwhile, the subjects received concurrent gemcitabine. Disease burden, body weight, performance status, hematologic and metabolic indices, time to progression, and overall survival of the subjects were monitored. After intravenous ascorbate treatment, mean plasma ascorbate trough levels were significantly higher than at baseline (1.46±0.02 vs. 0.78±0.09 mg/dL, i.e., 83 vs. 44μM, p < 0.001). Adverse events attributable to the drug combination were rare but included diarrhea (n = 4) and dry mouth (n = 6). Dose-limiting criteria were not met for this study. The mean survival of the subjects who completed at least two cycles (8 weeks) of the therapy was 13±2 months. Another completed clinical trial adding ascorbic acid to gemcitabine and erlotinib observed no increased toxicity in pancreatic cancer patients [80]. Accordingly, these data suggest that pharmacologic ascorbate administered concurrently with gemcitabine is well tolerated. Initial data from this small sample also suggest some efficacy, but further larger studies powered to determine efficacy should be conducted.
4.3.2Vitamin D
Conversion of quiescent to activated pancreatic stellate cells drives the severe stromal reaction that characterizes human pancreatic cancer. A recent study suggests that the vitamin D receptor (VDR) is expressed in stroma from human pancreatic tumors [81]. In a genetically engineered KPC-VDR–/– mouse model and an orthotopic transplant/allograft mouse model induced by orthotopic injection of 1×103 p53 2.1.1 cells into pancreas of 6- to 8-week-old FVB/n mice, treatment with the VDR ligand calcipotriol significantly reduced inflammatory markers and fibrosis in pancreatitis and human tumor stroma [81]. This study suggests that VDR could act as a transcriptional regulator of pancreatic stellate cells to reprise the quiescent state, leading to induced stromal remodeling, increased intratumoral gemcitabine concentration, reduced tumor volume, and a 57% increase in survival compared to chemotherapy alone [81]. This study also suggests that transcriptional reprogramming of tumor stroma could enhance the chemotherapeutic response and that vitamin D could be an adjunct agent in pancreatic cancer therapy [81]. Currently, a vitamin D derivative, paricalcitol, in combination with other chemotherapeutic agents (nivolumab, nab-paclitaxel, cisplatin, and gemcitabine), is being tested in an ongoing clinical trial of untreated metastatic pancreatic cancer patients (Table 1).
4.3.3Vitamin E
In KC mice, vitamin E δ-tocotrienol prolonged survival and delayed the progression of pancreatic intraepithelial neoplasia (PanIN) by inhibiting mutant Kras-driven pathways such as MEK/ERK, phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB, AKT), and NF-kB/p65 as well as B-cell lymphoma-extralarge (Bcl-xL), and p27. δ-Tocotrienol also induced apoptotic markers such as Bcl-2-associated X protein (Bax) and activated caspase-3, suggesting that it has chemopreventive potential [82]. A phase I pre-surgical trial was conducted to examine the safety, pharmacokinetic, and pharmacodynamic effects of vitamin E δ-tocotrienol in patients with pancreatic ductal neoplasia [83]. Soft gels encapsulating 99% pure vitamin E δ-tocotrienol (200–1600 mg daily taken orally for 2 weeks before pancreatic surgery) was well-tolerated, reached bioactive levels in blood, and significantly induced apoptosis in the neoplastic cells (measured by cleaved caspase-3) [83].
4.4Fish oil
Fish oil contains abundant ω-3 fatty acids (ω-3 FAs), which could decrease the production of pro-inflammatory cytokines. In a single-arm phase II trial, patients with advanced pancreatic adenocarcinoma were treated weekly with a ω-3 FA-rich lipid infusion (Lipidem, B. Braun Melsungen AG, Melsungen, Germany, up to 100 g/week) combined with gemcitabine chemotherapy until withdrawal or tumor progression. Twenty-three patients could be assessed for time to progression, overall survival, and complement activity. However, the study was not conclusive, and ω-3 FAs warrant further investigation in randomized clinical trials [84]. One study investigated the effects of perioperative immune-nutrition containing fish oil (Oral Impact®, Ajinomoto Pharma Co., Ltd, Tokyo, Japan) on cell-mediated immunity, T helper type 1 (Th1)/Th2 differentiation, and Th17 response after pancreaticoduodenectomy including pancreatic cancer [85, 86]. Thirty patients who underwent pancreaticoduodenectomy were divided into 3 arms: 10 patients in the perioperative group received preoperative immune-enhancing diets enriched in arginine, ω-3 FAs, and RNA for 5 days, which was prolonged postoperatively by enteral infusion; 10 patients in the postoperative group received early postoperative enteral infusion of the same enriched formula but with no artificial nutrition before the surgery; and 10 patients in the control group received total parenteral nutrition postoperatively. Immune responses were the primary endpoint, and the rate of infectious complications the secondary endpoint. Patients in the perioperative group had significantly increased concanavalin A- or phytohemagglutinin-stimulated lymphocyte proliferation and NK cell activation compared to the other two groups. Those patients also had significantly higher mRNA levels of T-bet, interferon-gamma (IFN-γ), related orphan receptor gammat (RORγt), and interleukin-17F (IL-17F). In the perioperative group, the rate of infectious complications was significantly lower than in the other groups. Perioperative treatment reduced stress-induced immunosuppression after a stressful major operative resection. These results suggest that modulation of Th1/Th2 differentiation and the Th17 response may play important roles in the immunologic effects of fish oil [85]. One open, randomized controlled trial investigated the effect of supplementary oral immune-nutrition (250–1000 mL daily, Oral Impact®, Nestlé) seven days before surgery on postoperative complications and length of hospital stay in pancreatic cancer patients [87], however, no difference were observed.
5Future prospective
The success of therapeutic strategies against pancreatic cancer will depend on defining the mechanisms of action of the chemopreventive agents through rigorous preclinical research. We must also enhance our ability to identify premalignant lesions, develop biomarkers for early diagnosis, and select the most effective personalized regimen for patients. Although in vitro cell culture studies provide fast tools for screening the efficacy of chemopreventive agents, in vivo animal studies, particularly genetically engineered animal models, can better recapitulate the complex tumor microenvironment of human pancreatic cancer. Therefore, preclinical research is much needed for producing clinically significant therapeutic options. For example, organoid models have shown huge potential in personalized medicine against pancreatic cancer [88–90], and patient-derived organoids provide a special system for discovering the pathways that drive each pancreatic tumor and for identifying novel therapies for personalized treatment decisions.
6Conclusion
Chemoprevention research continues to gain momentum worldwide as various epidemiological and preclinical findings provide evidence that natural products can help reduce pancreatic cancer risk. In the current review, we summarized results from human clinical intervention trials that used natural compounds under investigation for their efficacy against pancreatic cancer. Results from these trials suggest that better formulation of natural products to enhance their bioavailability is needed. The optimal window of intervention of natural products should also be carefully evaluated. Because of the extremely poor prognosis of pancreatic cancer, it is certainly valuable to investigate new methods of combating this disease, particularly for those at high risk, such as those with a family history of the disease and those who present with precancerous lesions such as PanIN, intraductal papillary mucinous neoplasm (IPMN), and mucinous cystic neoplasms (MCN). Accordingly, more preclinical work is needed to uncover mechanisms, increase absorption and bioavailability, and define an effective intervention window in which these natural products could have effects in vitro and in vivo, in order to produce more convincing results in a human clinical setting. Genetic animal models could provide a more relevant tumor microenvironment and tumor immunology of human pancreatic cancer patients. More research using genetic models and organoid models could translate into clinically significant chemoprevention of human pancreatic cancer.
Conflict of interest
No potential conflict of interest was disclosed.
Acknowledgments
This work was partially supported by NIH grant 5 R01 CA148818 and American Cancer Society, RSG-13-138-01— CNE to L.-S. Wang. We apologize to the investigators whose work could not be cited due to space limitation.
References
[1] | World Cancer Research Fund International: Pancreatic cancer statistics. Available at: http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/pancreatic-cancer-statistics. Accessed April 13, 2017. |
[2] | Stewart B.W. . WC. World Cancer Report Available at. http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014. Accessed April 6, 2017). |
[3] | Siegel R.L. , Miller K.D. , Jemal A. . Cancer Statistics. CA Cancer J Clin. (2017) ;67: (1):7–30. doi: 10.3322/caac.21387 |
[4] | Rahib L. , Smith B.D. , Aizenberg R. , Rosenzweig A.B. , Fleshman J.M. , Matrisian L.M. . Projecting cancer incidence and deaths to The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. (2913) ;74: (11):2913–21. doi: 10.1158/0008-5472.CAN-14-0155 |
[5] | Quante A.S. , Ming C. , Rottmann M. , Engel J. , Boeck S. , Heinemann V. , et al. Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer Med. (2016) ;5: (9):2649–56. doi: 10.1002/cam4.767 |
[6] | American Cancer Society: Pancreatic Cancer Risk Factors. Available at. https://www.cancer.org/cancer/pancreatic-cancer/causes-risks-prevention/risk-factors.html. Acessed April 13, 2017. |
[7] | Wiseman M. . The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Proc Nutr Soc. (2008) ;67: (3):253–6. doi: 10.1017/S002966510800712X |
[8] | Amayo A.A. , Kuria J.G. . Clinical application of tumour markers: A review. East African Medical Journal. (2009) ;86: (12 Suppl):S76–83. |
[9] | Humphris J.L. , Chang D.K. , Johns A.L. , Scarlett C.J. , Pajic M. , Jones M.D. , et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. (2012) ;23: (7):1713–22. doi: 10.1093/annonc/mdr561 |
[10] | Rossi M.L. , Rehman A.A. , Gondi C.S. . Therapeutic options for the management of pancreatic cancer. World J Gastroenterol. (2014) ;20: (32):2–59. doi: 10.3748/wjg.v20.i32.11142 |
[11] | Burris H.A. 3rd , Moore M.J. , Andersen J. , Green M.R. , Rothenberg M.L. , Modiano M.R. , et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. (1997) ;15: (6):2403–13. doi: 10.1200/jco.1997.15.6.2403 |
[12] | Von Hoff D.D. , Ervin T. , Arena F.P. , Chiorean E.G. , Infante J. , Moore M. , et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. (2013) ;369: (18):1691–703. doi: 10.1056/NEJMoa1304369 |
[13] | Conroy T. , Desseigne F. , Ychou M. , Bouche O. , Guimbaud R. , Becouarn Y. , et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. (2011) ;364: (19):1817–25. doi: 10.1056/NEJMoa1011923 |
[14] | Pelzer U. , Schwaner I. , Stieler J. , Adler M. , Seraphin J. , Dorken B. , et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: A phase III-study from the German CONKO-study group. Eur J Cancer. (2011) ;47: (11):1676–81. doi: 10.1016/j.ejca.2011.04.011 |
[15] | Oettle H. , Riess H. , Stieler J.M. , Heil G. , Schwaner I. , Seraphin J. , et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: Outcomes from the CONKO-003 trial. J Clin Oncol. (2014) ;32: (23):2423–9. doi: 10.1200/JCO.2013.53.6995 |
[16] | Wang-Gillam A. , Li C.P. , Bodoky G. , Dean A. , Shan Y.S. , Jameson G. , et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet. (2016) ;387: (10018):545–57. doi: 10.1016/S0140-6736(15)00986-1 |
[17] | Ruess D.A. , Gorgulu K. , Wormann S.M. , Algul H. . Pharmacotherapeutic Management of Pancreatic Ductal Adenocarcinoma: Current and Emerging Concepts. Drugs Aging. (2017) ;34: (5):331–57. doi: 10.1007/s40266-017-0453-y |
[18] | Stan S.D. , Singh S.V. , Brand R.E. . Chemoprevention strategies for pancreatic cancer. Nat Rev Gastroenterol Hepatol. (2010) ;7: (6):347–56. doi: 10.1038/nrgastro.2010.61 |
[19] | Boreddy S.R. , Srivastava S.K. . Pancreatic cancer chemoprevention by phytochemicals. Cancer Lett. (2013) ;334: (1):86–94. doi: 10.1016/j.canlet.2012.10.020 |
[20] | Categorical Glossary for the Flora of North America Project. http://fmhibd.library.cmu.edu/HIBD-DB/FNA/recordlist.php |
[21] | Wang L.S. , Burke C.A. , Hasson H. , Kuo C.T. , Molmenti C.L. , Seguin C. , et al. A phase Ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev Res (Phila). (2014) ;7: (7):666–74. doi: 10.1158/1940-6207.CAPR-14-0052 |
[22] | Wang L.S. , Arnold M. , Huang Y.W. , Sardo C. , Seguin C. , Martin E. , et al. Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: A phase I pilot study. Clin Cancer Res. (2011) ;17: (3):598–610. doi: 10.1158/1078-0432.CCR-10-1260 |
[23] | Pan P. , Skaer C.W. , Stirdivant S.M. , Young M.R. , Stoner G.D. , Lechner J.F. , et al. Beneficial regulation of metabolic profiles by black raspberries in human colorectal cancer patients. Cancer Prev Res (Phila). (2015) ;8: (8):743–50. doi: 10.1158/1940-6207.CAPR-15-0065 |
[24] | Pan P. , Skaer C.W. , Wang H.T. , Stirdivant S.M. , Young M.R. , Oshima K. , et al. Black raspberries suppress colonic adenoma development in ApcMin/+ mice: Relation to metabolite profiles. Carcinogenesis. (2015) ;36: (10):1245–53. doi: 10.1093/carcin/bgv117 |
[25] | Pan P. , Skaer C.W. , Wang H.T. , Oshima K. , Huang Y.W. , Yu J. , et al. Loss of free fatty acid receptor 2 enhances colonic adenoma development and reduces the chemopreventive effects of black raspberries in ApcMin/+ mice. Carcinogenesis. (2017) ;38: (1):86–93. doi: 10.1093/carcin/bgw122 |
[26] | Pan P. , Skaer C.W. , Wang H.T. , Kreiser M.A. , Stirdivant S.M. , Oshima K. , et al. Systemic metabolite changes in wild-type C57BL/6 mice fed black raspberries. Nutr Cancer. (2017) ;69: (2):299–306. doi: 10.1080/01635581.2017.1263748 |
[27] | Pan P. , Skaer C. , Wang H.T. , Tsai S. , Oshima K. , Huang Y-.W. , et al. Abstract LB- Black rapsberries induced protection in pancreatic cancer mouse models. Cancer Research. (2015) ;75: (15 Supplement):LB-268. |
[28] | Pan P. , Skaer C.W. , Wang H.-T. , Tsai S. , Oshima K. , Huang Y.-W. , et al. Abstract B Black raspberries inhibit pancreatic carcinogenesis by suppressing Raf/MEK/ERK/STAT3 signaling pathways and promoting apoptosis. Cancer Research.B. (2016) ;76: (24 Supplement):59. |
[29] | Westphalen C.B. , Olive K.P. . Genetically engineered mouse models of pancreatic cancer. Cancer J. (2012) ;18: (6):502–10. doi: 10.1097/PPO.0b013e31827ab4c4 |
[30] | Hingorani S.R. , Wang L. , Multani A.S. , Combs C. , Deramaudt T.B. , Hruban R.H. , et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. (2005) ;7: (5):469–83. doi: 10.1016/j.ccr.2005.04.023 |
[31] | Feig C. , Gopinathan A. , Neesse A. , Chan D.S. , Cook N. , Tuveson D.A. . The pancreas cancer microenvironment. Clin Cancer Res. (2012) ;18: (16):4266–76. doi: 10.1158/1078-0432.CCR-11-3114 |
[32] | Bosma M. , Schuler W. , Bosma G. . The scid mouse mutant. Curr Top Microbiol Immunol. (1988) ;137: :197–202. |
[33] | Mannal P.W. , Alosi J.A. , Schneider J.G. , McDonald D.E. , McFadden D.W. . Pterostilbene inhibits pancreatic cancer in vitro. J Gastrointest Surg. (2010) ;14: (5):873–9. doi: 10.1007/s11605-010-1164-4 |
[34] | Cedo L. , Castell-Auvi A. , Pallares V. , Macia A. , Blay M. , Ardevol A. , et al. Gallic acid is an active component for the anticarcinogenic action of grape seed procyanidins in pancreatic cancer cells. Nutr Cancer. (2014) ;66: (1):88–96. doi: 10.1080/01635581.2014.851714 |
[35] | Prasad R. , Vaid M. , Katiyar S.K. . Grape proanthocyanidin inhibit pancreatic cancer cell growth in vitro and in vivo through induction of apoptosis and by targeting the PI3K/Akt pathway. PLoS One. (2012) ;7: (8):e4306. doi: 10.1371/journal.pone.0043064 |
[36] | Prasad R. , Katiyar S.K. . Grape seed proanthocyanidins inhibit migration potential of pancreatic cancer cells by promoting mesenchymal-to-epithelial transition and targeting NF-kappaB. Cancer Lett. (2013) ;334: (1):118–26. doi: 10.1016/j.canlet.2012.08.003 |
[37] | Chung Y.C. , Huang C.C. , Chen C.H. , Chiang H.C. , Chen K.B. , Chen Y.J. , et al. Grape-seed procyanidins inhibit the in vitro growth and invasion of pancreatic carcinoma cells. Pancreas. (2012) ;41: (3):447–54. doi: 10.1097/MPA.0b013e318229da41 |
[38] | Golkar L. , Ding X.Z. , Ujiki M.B. , Salabat M.R. , Kelly D.L. , Scholtens D. , et al. Resveratrol inhibits pancreatic cancer cell proliferation through transcriptional induction of macrophage inhibitory cytokine-1. J Surg Res. (2007) ;138: (2):163–9. doi: 10.1016/j.jss.2006.05.037 |
[39] | Aggarwal B.B. , Kumar A. , Bharti A.C. Anticancer potential of curcum: Preclinical and clinical studies. Anticancer Research. (2003) ;23: (1a):363–98. |
[40] | Li N. , Chen X. , Liao J. , Yang G. , Wang S. , Josephson Y. , et al. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis. (2002) ;23: (8):1307–13. |
[41] | Ravindran J. , Prasad S. , Aggarwal B.B. . Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? The AAPS Journal. (2009) ;11: (3):495–510. doi: 10.1208/s12248-009-9128-x |
[42] | Sarkar F.H. , Li Y. , Wang Z. , Padhye S. . Lesson learned from nature for the development of novel anti-cancer agents: Implication of isoflavone, curcumin, and their synthetic analogs. Current Pharmaceutical Design. (2010) ;16: (16):1801–12. |
[43] | Kanai M. , Yoshimura K. , Asada M. , Imaizumi A. , Suzuki C. , Matsumoto S. , et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. (2011) ;68: (1):157–64. doi: 10.1007/s00280-010-1470-2 |
[44] | Dhillon N. , Aggarwal B.B. , Newman R.A. , Wolff R.A. , Kunnumakkara A.B. , Abbruzzese J.L. , et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. (2008) ;14: (14):4491–9. doi: 10.1158/1078-0432.CCR-08-0024 |
[45] | Epelbaum R. , Schaffer M. , Vizel B. , Badmaev V. , Bar-Sela G. . Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. (1137) ;62: (8):1137–41. doi: 10.1080/01635581.2010.513802 |
[46] | Parsons H.A. , Baracos V.E. , Hong D.S. , Abbruzzese J. , Bruera E. , Kurzrock R. . The effects of curcumin (diferuloylmethane) on body composition of patients with advanced pancreatic cancer. Oncotarget. (2016) ;7: (15):3–304. doi: 10.18632/oncotarget.7773 |
[47] | Anand P. , Kunnumakkara A.B. , Newman R.A. , Aggarwal B.B. Bioavailability of curcum Problems and promises Molecular pharmaceutics. (2007) ;4: (6):807–18. doi: 10.1021/mp700113r |
[48] | Shoba G. , Joy D. , Joseph T. , Majeed M. , Rajendran R. , Srinivas P.S. . Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Medica. (1998) ;64: (4):353–6. doi: 10.1055/s-2006-957450 |
[49] | Sato A. , Kudo C. , Yamakoshi H. , Uehara Y. , Ohori H. , Ishioka C. , et al. Curcumin analog GO-Y030 is a novel inhibitor of IKKbeta that suppresses NF-kappaB signaling and induces apoptosis. Cancer Sci. (1045) ;102: (5):1045–51. doi: 10.1111/j.1349-7006.2011.01886.x |
[50] | Bao B. , Ali S. , Banerjee S. , Wang Z. , Logna F. , Azmi A.S. , et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. (2012) ;72: (1):335–45. doi: 10.1158/0008-5472.CAN-11-2182 |
[51] | Dandawate P.R. , Vyas A. , Ahmad A. , Banerjee S. , Deshpande J. , Swamy K.V. , et al. Inclusion complex of novel curcumin analogue CDF and beta-cyclodextrin (2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharmaceutical Research. (2012) ;29: (7):1775–86. doi: 10.1007/s11095-012-0700-1 |
[52] | Bimonte S. , Barbieri A. , Palma G. , Luciano A. , Rea D. , Arra C. . Curcumin inhibits tumor growth and angiogenesis in an orthotopic mouse model of human pancreatic cancer. Biomed Res Int. (2013) ;2013: :810423. doi: 10.1155/2013/810423 |
[53] | Shevchenko I. , Karakhanova S. , Soltek S. , Link J. , Bayry J. , Werner J. , et al. Low-dose gemcitabine depletes regulatory T cells and improves survival in the orthotopic Panc02 model of pancreatic cancer. Int J Cancer. (2013) ;133: (1):98–107. doi: 10.1002/ijc.27990 |
[54] | Arshad A. , Al-Leswas D. , Al-Taan O. , Stephenson J. , Metcalfe M. , Steward W.P. , et al. Pooled survival and response data from phase III randomized controlled trials for gemcitabine-based regimes in the treatment of advanced pancreatic cancer. American Journal of Clinical Oncology. (2013) ;36: (4):411–4. doi: 10.1097/COC.0b013e3182124216 |
[55] | Olive K.P. , Tuveson D.A. . The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res. (2006) ;12: (18):5277–87. doi: 10.1158/1078-0432.CCR-06-0436 |
[56] | Hiura A. , Tsutsumi M. , Satake K. . Inhibitory effect of green tea extract on the process of pancreatic carcinogenesis induced by N-nitrosobis-(2-oxypropyl)amine (BOP) and on tumor promotion after transplantation of N-nitrosobis-(2-hydroxypropyl)amine (BHP)-induced pancreatic cancer in Syrian hamsters. Pancreas. (1997) ;15: (3):272–7. |
[57] | Bimonte S. , Leongito M. , Barbieri A. , Del Vecchio V. , Barbieri M. , Albino V. , et al. Inhibitory effect of (-)-epigallocatechin-3-gallate and bleomycin on human pancreatic cancer MiaPaca-2 cell growth. Infect Agent Cancer. (2015) ;10: :10–22. doi: 10.1186/s13027-015-0016-y |
[58] | Majima T. , Tsutsumi M. , Nishino H. , Tsunoda T. , Konishi Y. . Inhibitory effects of beta-carotene, palm carotene, and green tea polyphenols on pancreatic carcinogenesis initiated by N-nitorsobis(2-oxopropyl)amine in Syrian golden hamsters. Pancreas. (1998) ;16: (1):13–8. |
[59] | Qanungo S. , Das M. , Haldar S. , Basu A. . Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis. (2005) ;26: (5):958–67. doi: 10.1093/carcin/bgi040 |
[60] | Shankar S. , Ganapathy S. , Hingorani S.R. , Srivastava R.K. . EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. (2008) ;13: :440–52. |
[61] | Shankar S. , Marsh L. , Srivastava R.K. . EGCG inhibits growth of human pancreatic tumors orthotopically implanted in Balb C nude mice through modulation of FKHRL1/FOXO3a and neuropilin. Molecular and Cellular Biochemistry. (2013) ;372: (1-2):83–94. doi: 10.1007/s11010-012-1448-y |
[62] | Zhu Z. , Wang Y. , Liu Z. , Wang F. , Zhao Q. . Inhibitory effects of epigallocatechin-3-gallate on cell proliferation and the expression of HIF-1alpha and P-gp in the human pancreatic carcinoma cell line PANC-1. Oncology Reports. (2012) ;27: (5):1567–72. doi: 10.3892/or.2012.1697 |
[63] | Lyn-Cook B.D. , Rogers T. , Yan Y. , Blann E.B. , Kadlubar F.F. , Hammons G.J. . Chemopreventive effects of tea extracts and various components on human pancreatic and prostate tumor cells in vitro. Nutr Cancer. (1999) ;35: (1):80–6. doi: 10.1207/S1532791480-86 |
[64] | Anissi J. , El Hassouni M. , Ouardaoui A. , Sendide K. . A comparative study of the antioxidant scavenging activity of green tea, black tea and coffee extracts: A kinetic approach. Food Chem. (2014) ;150: :438–47. doi: 10.1016/j.foodchem.2013.11.009 |
[65] | Basu A. , Betts N.M. , Mulugeta A. , Tong C. , Newman E. , Lyons T.J. . Green tea supplementation increases glutathione and plasma antioxidant capacity in adults with the metabolic syndrome. Nutr Res. (2013) ;33: (3):180–7. doi: 10.1016/j.nutres.2012.12.010 |
[66] | Frei B. , Higdon J.V. . Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. The Journal of Nutrition. (2003) ;133: (10):3275s–84s. |
[67] | Guo Q. , Zhao B. , Shen S. , Hou J. , Hu J. , Xin W. . ESR study on the structure-antioxidant activity relationship of tea catechins and their epimers. Biochimica et Biophysica Acta. (1999) ;1427: (1):13–23. |
[68] | Nakagawa T. , Yokozawa T. . Direct scavenging of nitric oxide and superoxide by green tea. Food and chemical toxicology: An international journal published for the British Industrial Biological Research Association. (2002) ;40: (12):1745–50. |
[69] | Nanjo F. , Honda M. , Okushio K. , Matsumoto N. , Ishigaki F. , Ishigami T. , et al. Effects of dietary tea catechins on alpha-tocopherol levels, lipid peroxidation, and erythrocyte deformability in rats fed on high palm oil and perilla oil diets. Biol Pharm Bull. (1993) ;16: (11):1156–9. |
[70] | Newsome B.J. , Petriello M.C. , Han S.G. , Murphy M.O. , Eske K.E. , Sunkara M. , et al. Green tea diet decreases PCB 126-induced oxidative stress in mice by up-regulating antioxidant enzymes. The Journal of Nutritional Biochemistry. (2014) ;25: (2):126–35. doi: 10.1016/j.jnutbio.2013.10.003 |
[71] | Kostin S.F. , McDonald D.E. , McFadden D.W. . Inhibitory effects of (-)-epigallocatechin-3-gallate and pterostilbene on pancreatic cancer growth in vitro. J Surg Res. (2012) ;177: (2):255–62. doi: 10.1016/j.jss.2012.04.023 |
[72] | Braga M. , Bissolati M. , Rocchetti S. , Beneduce A. , Pecorelli N. , Di Carlo V. . Oral preoperative antioxidants in pancreatic surgery: A double-blind, randomized, clinical trial. Nutrition. (2012) ;28: (2):160–4. doi: 10.1016/j.nut.2011.05.014 |
[73] | Ji B.T. , Chow W.H. , Hsing A.W. , McLaughlin J.K. , Dai Q. , Gao Y.T. , et al. Green tea consumption and the risk of pancreatic and colorectal cancers. Int J Cancer. (1997) ;70: (3):255–8. |
[74] | Lin Y. , Kikuchi S. , Tamakoshi A. , Yagyu K. , Obata Y. , Kurosawa M. , et al. Green tea consumption and the risk of pancreatic cancer in Japanese adults. Pancreas. (2008) ;37: (1):25–30. doi: 10.1097/MPA.0b013e318160a5e2 |
[75] | Luo J. , Inoue M. , Iwasaki M. , Sasazuki S. , Otani T. , Ye W. , et al. Green tea and coffee intake and risk of pancreatic cancer in a large-scale, population-based cohort study in Japan (JPHC study). European Journal of Cancer Prevention: The Official Journal of the European Cancer Prevention Organisation (ECP). (2007) ;16: (6):542–8. doi: 10.1097/CEJ.0b013e32809b4d30 |
[76] | Wang J. , Zhang W. , Sun L. , Yu H. , Ni Q.X. , Risch H.A. , et al. Green tea drinking and risk of pancreatic cancer: A large-scale, population-based case-control study in urban Shanghai. Cancer Epidemiol. (2012) ;36: (6):e354–8. doi: 10.1016/j.cane2012.08.004 |
[77] | Zeng J.L. , Li Z.H. , Wang Z.C. , Zhang H.L. . Green tea consumption and risk of pancreatic cancer: A meta-analysis. Nutrients. (2014) ;6: (11):4640–50. doi: 10.3390/nu6114640 |
[78] | Du J. , Cieslak J.A. 3rd , Welsh J.L. , Sibenaller Z.A. , Allen B.G. , Wagner B.A. , et al. Pharmacological ascorbate radiosensitizes pancreatic cancer. Cancer Res. (2015) ;75: (16):3314–26. doi: 10.1158/0008-5472.CAN-14-1707 |
[79] | Welsh J.L. , Wagner B.A. , van’t Erve T.J. , Zehr P.S. , Berg D.J. , Halfdanarson T.R. , et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): Results from a phase I clinical trial. Cancer Chemother Pharmacol. (2013) ;71: (3). doi: 10.1007/s00280-013-2070-8 |
[80] | Monti D.A. , Mitchell E. , Bazzan A.J. , Littman S. , Zabrecky G. , Yeo C.J. , et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. (2012) ;7: (1):e29794. doi: 10.1371/journal.pone.0029794 |
[81] | Sherman M.H. , Yu R.T. , Engle D.D. , Ding N. , Atkins A.R. , Tiriac H. , et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. (2014) ;159: (1):80–93. doi: 10.1016/j.cell.2014.08.007 |
[82] | Husain K. , Centeno B.A. , Chen D.T. , Fulp W.J. , Perez M. , Zhang Lee G. , et al. Prolonged survival and delayed progression of pancreatic intraepithelial neoplasia in LSL-KrasG12D/+;Pdx-1-Cre mice by vitamin E delta-tocotrienol. Carcinogenesis. (2013) ;34: (4):858–63. doi: 10.1093/carcin/bgt002 |
[83] | Springett G.M. , Husain K. , Neuger A. , Centeno B. , Chen D.T. , Hutchinson T.Z. , et al. A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E delta-tocotrienol in Patients with Pancreatic Ductal Neoplasia. EBioMedicine. (2015) ;2: (12):1987–95. doi: 10.1016/j.ebiom.2015.11.025 |
[84] | Arshad A. , Chung W. , Isherwood J. , Steward W. , Metcalfe M. , Dennison A. . Restoration of mannose-binding lectin complement activity is associated with improved outcome in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega-3 fish oil. JPEN Journal of Parenteral and Enteral Nutrition. (2014) ;38: (2):214–9. doi: 10.1177/0148607113476304 |
[85] | Suzuki D. , Furukawa K. , Kimura F. , Shimizu H. , Yoshidome H. , Ohtsuka M. , et al. Effects of perioperative immunonutrition on cell-mediated immunity, T helper type 1 (Th1)/Th2 differentiation, and Th17 response after pancreaticoduodenectomy. Surgery. (2010) ;148: (3):573–81. doi: 10.1016/j.surg.2010.01.017 |
[86] | Aida T. , Furukawa K. , Suzuki D. , Shimizu H. , Yoshidome H. , Ohtsuka M. , et al. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreatoduodenectomy. Surgery. (2014) ;155: (1):124–33. doi: 10.1016/j.surg.2013.05.040 |
[87] | Gade J. , Levring T. , Hillingso J. , Hansen C.P. , Andersen J.R. . The effect of preoperative oral immunonutrition on complications and length of hospital stay after elective surgery for pancreatic cancer–a randomized controlled trial. Nutr Cancer. (2016) ;68: (2):225–33. doi: 10.1080/01635581.2016.1142586 |
[88] | Baker L.A. , Tiriac H. , Clevers H. , Tuveson D.A. . Modeling pancreatic cancer with organoids. Trends Cancer. (2016) ;2: (4):176–90. doi: 10.1016/j.trecan.2016.03.004 |
[89] | Hwang C.I. , Boj S.F. , Clevers H. , Tuveson D.A. . Preclinical models of pancreatic ductal adenocarcinoma. J Pathol. (2016) ;238: (2):197–204. doi: 10.1002/path.4651 |
[90] | Boj S.F. , Hwang C.I. , Baker L.A. , Chio I.I. , Engle D.D. , Corbo V. , et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. (2015) ;160: (1-2):324–38. doi: 10.1016/j.cell.2014.12.021 |



