Abstract
Background
Though significant progress has been made towards new diagnostic approaches for early detection of acute kidney injury (AKI) induced by different factors, there is still an urgent demand for a more specific and predictive biomarker for each type. The aim of this study is to unravel the potential diagnostic utility of circulating osteoprotegerin (OPG) in septic patients who developed AKI in the ICU, compared to cystatin C (a renal function maker) and KIM-1 (a kidney damage marker).
Methods
Eighty patients (male = 43, female = 37) with ages ranging from 42 to 46 years and with sepsis, 40 of whom developed AKI, and 30 healthy controls were enrolled in this prospective study.
Results
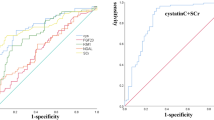
Results revealed significant progressive elevation of OPG, along with cystatinCand KIM-1, among sepsis, severe sepsis, and sepsis-AKI patients. The progression of OPG levels paralleled the deterioration of kidney and endothelial functions from sepsis to sepsis-AKI, revealed as progressively increased levels of serum Eselectin (15.3%), endothelin-1 (ET-1) (19.6%), and decreased nitric oxide (NO) (29.7%), associated with elevations of TNF-α (25.5%) and TGF-β (18%). Their comparative prognostic validity of sepsis-AKI was assessed using ROC analysis, which revealed that OPG, KIM-1, and cystatin C showed similar AUCs (0.827-0.83) but with different sensitivities, viz., 84%, 88%, and 92%, respectively. Although cystatin showed 82% specificity, OPG showed a higher, similar specificity to KIM-1 of 85%, indicating its potential function as a marker of renal damage such as KIM-1.
Conclusion
This study revealed a significant elevation of circulating OPG in septic patients with different levels of severity and those who progressed to AKI. Moreover, OPG showed a significant correlation to KIM-1 and cystatin, as well as conventional renal, inflammatory, and endothelial markers. Having a similar specificity to KIM-1, as evidenced by the ROC analysis, OPG has the potential to serve as a reliable biomarker of kidney damage in cases of sepsis-AKI.
Similar content being viewed by others
Abbreviations
- AKI:
-
acute kidney injury
- GFR:
-
glomerular filtration rate
- ET-1:
-
endothelin-1
- DN:
-
diabetic nephropathy
- ECM:
-
extracellular matrix
- ICU:
-
intensive care unit
- ICAM-1:
-
intercellular adhesion molecule
- IFN-γ:
-
interferon gamma
- IL:
-
interleukin
- OPG:
-
osteoprotegerin
- KIM-1:
-
kidney injury molecule 1
- NGAL:
-
neutrophil gelatinase-associated lipocalin
- NO:
-
nitric oxide
- iNOS:
-
inducible NO synthase
- eNOS:
-
endothelial NO synthase
- ROC:
-
receiver operating curve
- RANKL:
-
receptor activator of NF-kappa B ligand
- TNF-α:
-
tumor necrosis factor-α
- TNF-R tumor:
-
necrosis factor-α receptor
- TGF-β:
-
transforming growth factor-β
- TNFSF:
-
tumor necrosis factor-α superfamily
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- TWEAK:
-
TNF-like weak inducer of apoptosis
- LPS:
-
lipopolysaccharide
- LBP:
-
lipopolysaccharide binding protein
- WBC:
-
white blood count
- VCAM-1:
-
vascular cell adhesion molecule
References
Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS): a prospective study. JAMA 1995; 273: 117–23.
Alobaidi R, Basu RK, Goldstein SL, Bagshaw SM. Sepsis-associated acute kidney injury. Semin Nephrol 2015; 35: 2–11.
Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005; 365: 1231–8.
Schiffl H, Lang SM. Update on biomarkers of acute kidney injury: moving closer to clinical impact? Mol Diagn Ther 2012; 16: 199–207.
Wagener G, Jan M, Kim M, et al. Association between increases in urinary neutrophil gelatinaseassociated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology 2006; 105: 485–91.
de Boer IH, Katz R, Cao JJ, et al. Cystatin C, albuminuria, and mortality among older adults with diabetes. Diabetes Care 2009; 32: 1833–8.
Delanaye P, Cavalier E, Radermecker RP, et al. Cystatin C or creatinine for detection of stage 3 chronic kidney disease in anorexia nervosa. Nephron Clin Pract 2008; 110: c158–63.
Bonventre J. Kidney injury molecule 1: a urinary biomarker and much more. Nephrol Dial Transplant 2009; 24: 3265–8.
Huang Y, Don-Wauchope AC. The clinical utility of kidney injury molecule 1 in the prediction, diagnosis and prognosis of acute kidney injury: a systematic review. Inflamm Allergy Drug Targets 2011; 10: 260–71.
Briguori C, Quintavalle C, Donnarumma E, Condorelli G. Novel biomarkers for contrast-induced acute kidney injury. Biomed Res Int 2014; 2014: 568738.
Truneh A, Sharma S, Silverman C, et al. Temperature-sensitive differential affinity of TRAIL for its receptors: DR5 is the highest affinity receptor. J Biol Chem 2000; 275: 23319–25.
Lorz C, Benito-Martin A, Boucherot A, et al. The death ligand TRAIL in diabetic nephropathy. J Am Soc Nephrol 2008; 19: 904–14.
Rimondi E, Secchiero P, Quaroni A, Zerbinati C, Capitani S, Zauli G. Involvement of TRAIL/TRAIL receptors in human intestinal cell differentiation. J Cell Physiol 2006; 206: 647–54.
Dellinger R, Levy M, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39: 165–228.
Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:31.
Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. In response to the recently released 2012 KDIGO (Kidney disease: improving global outcomes). Am J Kidney Dis 2013; 61: 649–72.
Pelte CH, Chawla LS. Novel therapeutic targets for prevention and therapy of sepsis associated acute kidney injury. Curr Drug Targets 2009; 10: 1205–11.
Majumdar A. Sepsis-induced acute kidney injury. Ind J Crit Care Med 2010; 14: 14–21.
Al-Lamki RS, Wang J, Skepper JN, Thiru S, Pober JS, Bradley JR. Expression of tumor necrosis factor receptors in normal kidney and rejecting renal transplants. Lab Invest 2001; 81: 1503–15.
Morena M, Jaussent I, Dupuy AM, et al. Osteoprotegerin and sclerostin in chronic kidney disease prior to dialysis: potential partners in vascular calcifications. Nephrol Dial Transplant 2015, pii: gfv081. [Epub ahead of print].
Benito-Martin A, Ucero AC, Zubiri I, et al. Osteoprotegerin in exosome-like vesicles from human cultured tubular cells and urine. PLoS One 2013; 8: e72387.
Lorz C, Benito-Martin A, Boucherot A, et al. The death ligand TRAIL in diabetic nephropathy. J Am Soc Nephrol 2008; 19: 904–14.
Dutta J, Fan Y, Gupta N, Fan G, Gelinas C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene 2006; 25: 6800–16.
Karacay B, Sanlioglu S, Griffith T S, Sandler A, Bonthius DJ. Inhibition of the NF-kappaB pathway enhances TRAIL-mediated apoptosis in neuroblastoma cells. Cancer Gene Ther 2004; 11: 681–90.
Takayanagi H, Ogasawara K, Hida S, et al. T-cell-mediated regulation of osteoclastogenesis by signaling cross-talk between rankl and ifn-γ. Nature 2000; 408: 600–5.
Sandberg WJ, Yndestad A, Oie E, et al. Enhanced T-cell expression of rank ligand in acute coronary syndrome: possible role in plaque destabilization. Arterioscler Thromb Vasc Biol 2006; 26: 857–63.
Abedin M, Omland T, Ueland T, et al. Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas heart study). Am J Cardiol 2007; 99: 513–8.
Weitzmann MN. The role of inflammatory cytokines, the RANKL/OPG axis, and the immunoskeletal interface in physiological bone turnover and osteoporosis. Scientifica 2013; 2013: 125705.
Kim S, Mi L, Zhang L. Specific elevation of DcR3 in sera of sepsis patients and its potential role as a clinically important biomarker of sepsis. Diagn Microbiol Infect Dis 2012; 73: 312–7.
Karlsson S, Heikkinen M, Pettilä V, et al. Finnsepsis study group. Predictive value of procalcitonin decrease in patients with severe sepsis: a prospective observational study. Crit Care 2010; 14: R205.
Halacova M, Kotaska K, Kukacka J, et al. Serum cystatin C level for better assessment of glomerular filtration rate in cystic fibrosis patients treated by amikacin. J Clin Pharm Ther 2008; 33: 409–17.
Vaidya VS, Ford GM, Waikar SS, et al. A rapid urine test for early detection of kidney injury. Kidney Int 2009; 76: 108–14.
Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002; 62: 237–44.
Haase M, Bellomo R, Devarajan P, et al. Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg 2009; 88: 124–30.
Perianayagam MC, Seabra VF, Tighiouart H, Liangos O, Jaber BL. Serum cystatin C for prediction of dialysis requirement or death in acute kidney injury: a comparative study. Am J Kidney Dis 2009; 16: 1025–33.
Liangos O, Perianayagam MC, Vaidya VS, et al. Urinary N-acetylbeta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol 2007; 16: 904–12.
Nejat M, Pickering JW, Walker RJ, Endre ZH. Rapid detection of acute kidney injury by plasma cystatin C in the intensive care unit. Nephrol Dial Transplant 2010; 25: 3283–9.
Kim DJ, Kang HS, Choi HS, et al. Serum cystatin C level is a useful marker for the evaluation of renal function in patients with cirrhotic ascites and normal serum creatinine levels. Korean J Hepatol 2011; 17: 130–8.
Martensson J, Martling CR, Oldner A, Bell M. Impact of sepsis on levels of plasma cystatin C in AKI and non-AKI patients. Nephrol Dial Transplant 2012; 27: 576–81.
Ortu˜no-Andériz F, Cabello-Clotet N, Vidart-Simón N, Postigo-Hernández C, Domingo-Marín S, Sánchez-García M. Cystatin C as an early marker of acute kidney injury in septic shock. Rev Clin Esp 2015; 215: 83–90.
Zhongheng Z. Biomarkers, diagnosis and management of sepsisinduced acute kidney injury: a narrative review. Heart Lung Vessels 2015; 7: 64–73.
Kulcsar-Jakab E, Petho Z, Pap Z, et al. Cystatin C as a potential predictor of osteoprotegerin levels in healthy men, a cross-sectional, observational study. BMC Musculoskelet Disord 2015; 16:227.
Fekih O, Triki H, Triki S, et al. Osteoprotegerin as a marker of cardiovascular risk in children and adolescents with type 1 diabetes. Pediatr Diabetes 2017; 18: 230–6.
Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332: 411–5.
Er H, Evereklioglu C, Cumurcu T, et al. Serum homocysteine level is increased and correlated with ET-1 and nitric oxide in Behcet’s disease. Br J Ophthalmol 2002; 86: 653–7.
Wu X, Guo R, Wang Y, Cunningham PN. The role of ICAM-1 in endotoxin-induced acute renal failure. Am J Physiol Renal Physiol 2007; 293: F1262–71.
Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 2002; 62: 1539–49.
Pigott R, Dillon LP, Hemingway IH, Gearing AJH. Soluble forms of E-selectin, ICAM-1 and VCAM-1 are present in the supernatants of cytokine activated cultured endothelial cells. Biochem Biophys Res Commun 1992; 187: 584–9.
Ficek R, Kokot F, Chudek J, Adamczak M, Ficek J, Wiecek A. Plasma concentrations of tumor necrosis factor alpha may predict the outcome of patients with acute renal failure. Kidney Blood Press Res 2006; 29: 203–9.
Sonkar GK, Usha Singh RG. Evaluation of serum tumor necrosis factor alpha and its correlation with histology in chronic kidney disease, stable renal transplant and rejection cases. Saudi J Kidney Dis Transpl 2009; 20: 1000–4.
Wan J, Bagshaw S, Langenberg B, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: what do we really know? Crit Care Med 2008; 36: S198–203.
Guan Q, Nguan CY, Du C. Expression of transforming growth factor-beta 1 limits renal ischemia-reperfusion injury. Transplantation 2010; 89: 1320–7.
Sadik NA, Mohamed WA, Ahmed MI. The association of receptor of advanced glycated end products and inflammatory mediators contributes to endothelial dysfunction in a prospective study of acute kidney injury patients with sepsis. Mol Cell Biochem 2012; 359: 73–81.
Alejandro V, Scandling Jr. JD, Sibley RK, et al. Mechanisms of filtration failure during postischemic injury of the human kidney. A study of the reperfused renal allograft. J Clin Invest 1995; 95: 820–31.
Noiri E, Peresieni T, Miller F, Goligorsky MS. In vivo targeting of inducible NO synthase with oligodeoxynucleotides protects rat kidney against ischemia. J Clin Invest 1996; 97: 2377–83.
Goligorsky MS, Brodsky SV, Noiri E. Nitric oxide in acute renal failure: NOS versus NOS. Kidney Int 2002; 61: 855–61.
Schneider R, Raff U, Vornberger N, et al. L-Arginine counteracts nitric oxide deficiency and improves the recovery phase of ischemic acute renal failure in rats. Kidney Int 2003; 64: 216–25.
Kwon O, Hong SM, Ramesh G. Diminished NO generation by injured endothelium and loss of macula densa nNOS may contribute to sustained acute kidney injury after ischemia-reperfusion. Am J Physiol Renal Physiol 2009; 296: F25–33.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Schaalan, M., Mohamed, W. Predictive ability of circulating osteoprotegerin as a novel biomarker for early detection of acute kidney injury induced by sepsis. Eur Cytokine Netw 28, 52–62 (2017). https://doi.org/10.1684/ecn.2017.0393
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1684/ecn.2017.0393