-
PDF
- Split View
-
Views
-
Cite
Cite
Susan Mills, R. Paul Ross, Colin Hill, Bacteriocins and bacteriophage; a narrow-minded approach to food and gut microbiology, FEMS Microbiology Reviews, Volume 41, Issue Supp_1, August 2017, Pages S129–S153, https://doi.org/10.1093/femsre/fux022
Close - Share Icon Share
Abstract
Bacteriocins and bacteriophage (phage) are biological tools which exhibit targeted microbial killing, a phenomenon which until recently was seen as a major drawback for their use as antimicrobial agents. However, in an age when the deleterious consequences of broad-spectrum antibiotics on human health have become apparent, there is an urgent need to develop narrow-spectrum substitutes. Indeed, disruption of the microbial communities which exist on and in our bodies can generate immediate and long-term negative effects and this is particularly borne out in the gut microbiota community whose disruption has been linked to a number of disorders reaching as far as the brain. Moreover, the antibiotic resistance crisis has resulted in our inability to treat many bacterial infections and has triggered the search for damage-limiting alternatives. As bacteriocins and phage are natural entities they are relatively easy to isolate and characterise and are also ideal candidates for improving food safety and quality, forfeiting the need for largely unpopular chemical preservatives. This review highlights the efficacy of both antimicrobial agents in terms of gut health and food safety and explores the body of scientific evidence supporting their effectiveness in both environments.
INTRODUCTION
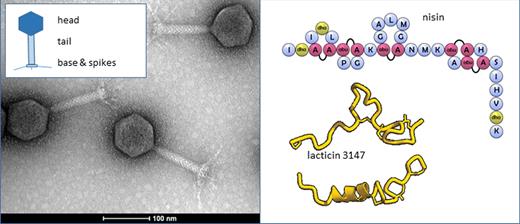
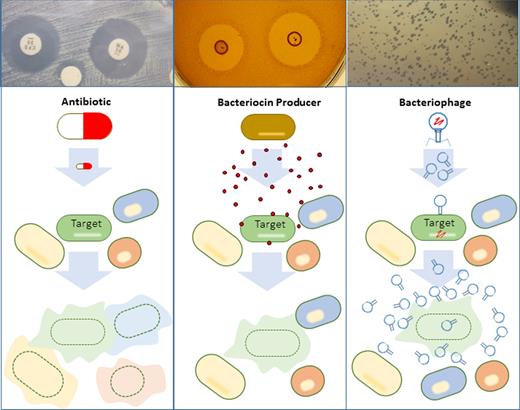
Every ecosystem, including the human body and food, is a battleground where biological entities compete for valuable resources. These biological entities deploy strategies to ensure their own survival and to promote competition with other, often related species. If we want to shape microbial ecosystems in food, or in the gut, we can look to the natural biological agents used by the existing microbiota, such as bacteriocins and bacteriophage (phage) as antimicrobial tools with potential biotechnological applications. Bacteriocins are gene-encoded antimicrobial peptides produced by bacteria, and phage are viruses which infect and can kill bacterial cells (Fig. 1). Both were discovered in the first half of the 20th century but their potential as antimicrobial agents was overshadowed by the discovery of broad-spectrum antibiotics and food preservatives. The generally narrow inhibition spectrums of both bacteriocins and phage were seen as significant drawbacks to their applications. However, with the hindsight of a century of research and discovery it has become apparent that perhaps that very attribute, their narrow spectrums of activity, may well make them the ideal candidates in the frontline battle against problematic bacteria. One of the key drivers of this change in mind set is our growing appreciation of the importance of the gut microbiota to overall health and the potentially negative consequences of its disruption, which has been associated with a host of immunological, metabolic and neurological disorders (Li et al. 2016). It is now accepted that the collateral damage affected by broad-spectrum antibiotics on the gut microbiota may well be a factor in many diseases (Ferrer et al.2016; Ianiro, Tilg and Gasbarrini 2016). More specifically, disruption of the gut microbiota as a consequence of antibiotic administration is a major contributing factor in Clostridium difficile infection (De La Cochetière et al.2008; Theriot et al.2014). In this regard, narrow-spectrum antimicrobials which exhibit minimal collateral damage to the overall microbiota are far more desirable (Fig. 2). Moreover, bacteriocins and phage offer potential as tools to re-shape the microbiota by exclusively depleting specific targets and allowing for favourable shifts in bacterial abundance and diversity.
Bacteriophage (left) and bacteriocins (right). Bacteriophage (in this case an Escherichia coli Myoviridae) are composed of a head containing the nucleic acid, a tail and a base plate with spikes. Other phage can have collars and other structural features. The bacteriocins shown include nisin, showing the complex post-translational modifications, and lacticin 3147, a two-component highly modified lantibiotic.
Off-target consequences of antibiotics, bacteriocins and phage on microbial communities. As well as killing its target the antibiotic kills the surrounding microbial community. In contrast, bacteriocins and phage do not alter the surrounding microbial community but kill the specific target only.
The antibiotic resistance crisis further adds to the appeal of bacteriocins and phage. Indeed, the emergence of antibiotic-resistant bacteria has rendered several bacterial infections extremely difficult, if not impossible, to treat and the development of new antibiotics has been almost at a standstill; the discovery of the broad-sprectrum antibiotic teixobactin in a screen of uncultured soil bacteria in early 2015 (Ling et al.2015) was hailed as the first new antibiotic to be discovered in 30 years (Knapton 2016). In contrast, phage have been described as the most abundant entities on the planet (Weinbauer 2004) and it has been suggested that 30%–99% of the Bacteria and Archaea produce at least one bacteriocin (Klaenhammer 1988; Riley 1998). Antibiotics have also been commonly used in the food chain to promote health and animal growth, a practice which is increasingly under threat from regulatory bodies concerned with the impact on human health.
In terms of food preservation, chemical preservatives are commonly used to inhibit bacterial growth. However, many of these exhibit side effects which can range from mild to life threatening (Sharma 2015). For example, increased consumption of nitrites, which are particularly used in cured meat products to inhibit food spoilage and pathogenic bacteria, has been identified as a potential risk factor for gastric cancer (Song, Wu and Guan 2015). Thus, a ‘preservative-free diet’ is considered best practice (Sharma 2015). Consumers are aware of these trends and naturally favour minimally processed foods which are also low in salt and sugar but are safe, tasty and have long shelf lives. However, such demands intrinsically lead to increased risk of food pathogens and spoilage. Food spoilage as a result of bacterial contamination results in significant economic losses for food producers and processors on an annual basis, particularly for the ready-to-eat (RTE) food sectors. In addition, food poisoning as a result of bacterial contamination is an ongoing issue with EFSA reporting a total of 4362 outbreaks in 2015 in 32 European countries, the majority caused by bacteria (EFSA 2016).
Bacteriocins and phage are non-toxic to human cells and do not interfere with the sensory quality of foods. It is hardly surprising therefore that several phage-based products are available for food biocontrol, including ListShield (Intralytix Inc, Baltimore, USA) which targets Listeria monocytogenes and Salmonellex (Microes BV, Wageningen, The Netherlands) which targets Salmonella. While phage therapy for the treatment of human infections has been routinely practiced for decades in Russia, Georgia and Poland (Kutter et al.2010; Kutateladze 2015; Chanishvilli 2016), it is currently undergoing a revival in Western Europe where scientists, medical practitioners and biotech companies are working to bring it into mainstream medical practice.
Despite the potential offered by bacteriocins, only two are widely used commercially for food safety applications. One is the Lactococcus lactis bacteriocin nisin which is produced by many companies as concentrated fermentate powder or otherwise (e.g. Nisaplin, marketed by Dupont, Delaware, USA; Nisin Vega marketed by VEGA, Zhejiang, China). The second is a Carnobacterium maltaromaticum bacteriocin carnocyclin A which comes as a cell-free culture supernatant (Micocin®, Griffith Laboratories Scarborough, Canada). Nisin, which exhibits antimicrobial activity against a broad range of Gram-positive bacteria including Clostridium botulinum, Listeria monocytogenes and Staphylococcus aureus, has been approved as a safe food additive by the Joint Food and Agriculture Organization/World Health Organization (FAO/WHO) since 1969 (Shin et al.2015). It has been on the European food additives list since the early 1980s where it is assigned the number E234 (EEC 1983). It has GRAS (generally recognised as safe) status from the Food and Drug Administration (FDA) since the late 1980s (Federal Register 1988) and it is licensed as a biopreservative in over 50 countries (Alvarez-Sieiro et al.2016). Micocin® has been approved as a biopreservative in the USA and Canada for inhibition of L. monocytogenes. Bacteriocins may also represent some of the crucial antimicrobial ingredients in commercially available food safety fermentates along with other ingredients such as organic acids. For example, the fermentate MICROGARD (DuPont), recommended for the shelf-life protection of dairy foods and filled chocolate confectionery, contains a bacteriocin produced by Propionibacterium (Martinez, Rodríguez and Suárez 2016). A fermentate powder produced from the pediocin-producing strain Pediococcus acidilactici (ALTA 2351, Kerry Biosciences, Ireland) can be used to protect meat products from L. monocytogenes contamination (López-Cuellar, Rodríguez-Hernández and Chavarría-Hernández 2016). To date, biomedical applications of bacteriocins have not been developed to any significant extent. Yet between 2004 and 2015, bacteriocins have been the central topic of 429 published papers and 245 granted patents where nearly 40% of the research was focused on a range of biomedical applications (e.g. systemic infections, cancer, skin care, oral care and contraception, where in the latter case certain bacteriocins have been shown to reduce sperm motility (Kumar et al.2012; Kaur et al. 2013)) and ∼30% focused on food preservation (López-Cuellar, Rodríguez-Hernández and Chavarría-Hernández 2016). Our deepening understanding of bacteriocin functionality clearly suggests that these antimicrobial peptides have as yet an untapped potential and can be particularly potent when used in conjunction with other antimicrobial hurdles.
In this review, we critically assess the role of bacteriocins and phage for improving the quality and safety of food from farm to fork and present the latest innovations which aim to harness their full potential. We also assess the potential for bacteriocins and phage in gut health applications, from treating specific infections to modulating the microbiota towards the treatment and management of disease.
THE BIOLOGY OF BACTERIOCINS AND PHAGE
Bacteriocins
Bacteriocins are a diverse group of ribosomally produced antimicrobial peptides. Some bacteriocins undergo extensive post-translational modifications, an attribute which, together with their mode of action, has been used for their classification (Cotter, Hill and Ross 2005). The most recent classification scheme presented by Alvarez-Sieiro et al. (2016) suggests three classes based on the mechanism of biosynthesis and biological activity of lactic acid bacteria (LAB) bacteriocins, but the scheme is also valid for bacteriocins from other microorganisms. Class I, also known as the RiPPs (ribosomally produced and post-translationally modified peptides) have molecular masses of <10 kDa and represent all bacteriocins which undergo posttranslational modifications resulting in unusual amino acids and structures, including lanthionines, glycosylation and/or heterocycles. Class I is further divided into six subclasses consisting of the lanthipeptides (which is further divided into four types), cyclised peptides, linearazol(in)e-containing peptides (LAPs), sactibiotics, glycocins and lasso peptides. Class II represents the unmodified peptides of <10 kDa in size of which four subclasses exist: pediocin-like, two-peptides, leaderless and non-pediocin-like single peptides. Class III are thermo-labile and larger than 10 kDa and are subdivided into the bacteriolysins and the non-lytic bacteriocins.
The genes responsible for bacteriocin production normally exist as clusters consisting of structural genes, modification and maturation genes (in the case of RiPPs), transport and immunity genes. Immunity genes ensure that the producing strain is protected from the antimicrobial activity of its own bacteriocin. The mode of action varies from class to class (Alvarez-Sieiro et al.2016). Most members of classes I and II cause cell death through pore formation in the target cell after binding to specific receptors. For example, lipid II is the target receptor for several lantibiotics (Breukink and de Kruijff 2006). Members of the class IIa or pediocin-like bacteriocins have been shown to cause pore formation by binding to the mannose phosphotransferase system (Man-PTS) in the target cell membrane (Diep et al.2007). New bacteriocin receptors continue to be identified and recently UppP, a membrane spanning protein, was confirmed as the receptor for the class IIb bacteriocins, lactococcin G and enterocin 1071 (Kjos et al.2014). The class III bacteriolysins, such as zoocin A and enterolysin A, cause cell death by degrading peptidoglycan (Simmonds et al.1996; Khan, Flint and Yu 2013), whereas the non-lytic class III bacteriocins can disrupt bacterial growth by interfering with cellular processes (Alvarez-Sieiro et al.2016). An example of this is caseicin, produced by Lactobacillus casei, which interferes with protein and DNA biosynthesis in the target cell although this is not considered its primary mode of action (Müller and Radler 1993). Dysgalacticin produced by Streptococcus pyogenes has been shown to inhibit sugar uptake by targeting the glucose- and/or Man-PTS which also perturbs membrane integrity resulting in membrane leakage (Swe et al.2009).
Phage
This review will focus on tailed phage, which belong to the order Caudovirales and harbour double-stranded DNA genomes inside a polyhedral head (most frequently icosahedral) attached to a tail (Veesler and Cambillau 2011). There are three families based on tail morphology: Myoviridae (long, contractile tail), Siphoviridae (long, non-contractile tail) and Podoviridae (short tail). They generally range in size from 22 to 200 nm in length (Patel et al.2015). Host specifity is governed by proteins at the end of the tail fibre which recognise specific molecules on the bacterial surface (Guttman, Raya and Kutter 2005; Ackermann 2009). Phage are obligate intracellular parasites, mainly replicating via the lytic or lysogenic lifecycle. In the case of lysogeny, the phage does not cause cell lysis but integrates into the bacterial genome and replicates in concert with the host genome until a lytic event is triggered. In contrast, the exclusively lytic lifecycle is the fundamental process behind phage therapy and phage biocontrol with an endpoint resulting in death of the host cell and release of lytic phage.
Due to the narrow host range of phage (strain specific in most cases), cocktails consisting of two or more phage can be used to broaden the antimicrobial spectrum and reduce the risk of phage resistance.
FOOD
Bacteriocins
While bacteriocins can be produced by a range of Gram-positive and Gram-negative bacteria, LAB bacteriocins are of particular interest to the food industry for a number of reasons. First, members of the LAB group have a history of safe use as starter cultures in food fermentations and many possess GRAS and Qualified Presumption of Safety status (Alvarez-Sieiro et al.2016). Due to the health benefits associated with many LAB members, they are generally well received amongst consumers. Along with being non-toxic to eukaryotic cells, LAB bacteriocins are sensitive to gut proteases such as trypsin, chymotrypsin and pancreatin complex; thus, they are predicted to have minimum impact on the gut microbiota (Egan et al.2016). Bacteriocins are extremely potent, exhibiting killing activity at nanomolar concentrations (Jenssen, Hamill and Hancock 2006). Moreover, their gene-encoded nature makes them highly amenable to bioengineering strategies (Field et al. 2015). This has enabled the generation of different nisin variants with enhanced antimicrobial activity (Molloy et al.2013) and improved physico-chemical properties (Rollema et al.1995; Yuan et al.2004; Rouse et al.2012).
Combining bacteriocins with other preservation methods and hurdles is the ideal approach to harnessing their full antimicrobial potential (Mills et al.2011) enabling them to target even Gram-negative pathogens once the outer membrane has been destabilised (Prudêncio, dos Santos and Dantas Vanetti 2015).
There are four main methods by which bacteriocins can be used to improve food quality and safety, examples of which are outlined in Table 1 and Fig. 3: (i) direct addition of pure or partially purified bacteriocin or cell-free supernatant to the food product; (ii) direct addition of a bacteriocin-producing culture; (iii) the use of bacteriocins in antimicrobial packaging; (iv) the use of bacteriocins as sanitisers.
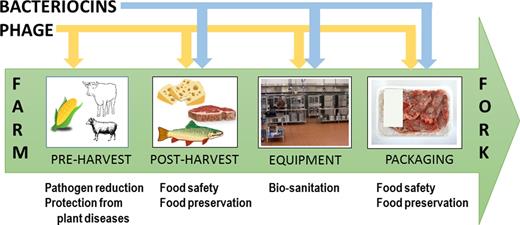
Bacteriocin and phage applications for protecting food against pathogens and spoilage bacteria from farm to fork.
Bacteriocin applications for food safety and quality purposes.
| Bacteriocin . | ||||||
|---|---|---|---|---|---|---|
| Bacteriocin (+ additional hurdle) . | Producer . | Food type . | Target microorganism . | Reduction (storage time/temp) . | Pros and cons . | Reference . |
| Lactoccin BZ (1600 AU/ml) | Lactococus lactis | Fresh beef | Listeria innocua | 6 logs (6 d/4–5°C) | Pros: Bacteriocin activity unaffected by meat components or pH of meat | Yildirim et al. (2016) |
| SH01 (1000 AU/g) | Enterococcus faecium | Ground beef | Listeria monocytogenes | 2.33 logs (8 d/20 oC) | Pros: Bacteriocin activity increases with temperatures up to 20oC | Kim, Jung and Kim (2015) |
| Cons: Bacteriocin sensitive to protease activity | ||||||
| Salivaricin KLD (10%) | Lactobacillus salivarius | Creamy filling | Bacillus cereusEnterococcus faecalisPseudomonas stutzeriStaphylococcus hominisStenotrophomonas sp. | 66.9%100%100%25.8%100% (3 d/37°C) | Pros: Bacteriocin stable at high temperature (100°C) and pH 3–10Active against Gram negativesCons: Bacteriocin sensitive to protease activity | Therdtatha et al. (2016) |
| Aureocin A53 (256 AU/ml) | Staphylococcus aureus | Skimmed milk | Listeria monocytogenes | 7.7 logs (7 d/4oC) | Pros: Highly resistant to proteolytic degradation | Fagundes et al. (2016) |
| Cons: Producing strain is a recognised human pathogen and produces two non-classical enterotoxins and a haemolysin | ||||||
| Enterocin AS-48 (250 μg/ml) | Enterococcus faecalis | Sardines (vacuum packed) | Endogenous staphylococci | >1 log (6 d/5°C) | Pros: Decreased levels of biogenic amines cadaverine, putrescine, tyramine and histamine by several fold | Ananou et al. (2014) |
| Cons: Antimicrobial activity against spoilage microbiota not highly effective because most of the spoilage microbiota are Gram negatives | ||||||
| Method of AS-48 application (immersion of | ||||||
| fish in bacteriocin) may be inadequate to ensure sufficiently high bacteriocin concentration on the food | ||||||
| Leukocin K7 (80 AU/ml) + glycine (5 mg/ml) | Leuconostoc mesenteroides | Milk | Listeria monocytogenes | 3 logs (1 d/4 oC) | Pros: Prevented emergence of resistant mutants over 7-day study period | Shi et al. (2016) |
| Nisin +garlic (2.08 mg/g garlic sprout) in phosphptidylcholine nanoliposomes | Lactococcus lactis | Milk | Listeria monocytogenesSalmonella enteriditisEscherichia coliStaphylococcus aureus | ∼6 logs (10 h/37°C)∼3–4 log∼3–4 logs∼5 logs (24 h/37°C) | Pros: Nisin and garlic more effective than either antimicrobial aloneCons: Garlic extract could influence the flavour of the milk. | Pinilla and Brandelli (2016) |
| Enterocin AS-48 (50 μg/g) + high hydrostatic pressure (600 MPa, 8 min) | Enterococcus faecalis A-48-32 | Cherimoya pulp | Artificially contaminated with natural microbiota | >6 logs(1 d/5°C) | Pros: Combined treatment more effective than either treatment alone and reduced Gram positives and Gram negatives.Cons: Microbial counts increased significantly after 15 days of storage | Pérez Pulido et al. (2015) |
| Bacteriocin-producing culture | ||||||
| Culture | Bacteriocin | Food type | Target microorganism | Reduction (storage time/temp) | Pros and cons | Reference |
| Lactobacillus sakei subsp. sakei 2a | Sakacin | Cheese spread | Listeria monocytogenes | ∼3 logs (28 d/15°C) | Pros: Bacteriocin production in cheese during storage was confirmed via expression of bacteriocin genesCons: Impact of bacteriocin producer on cheese flavour to be confirmed | Martinez et al. (2015) |
| Enterococcus faecalis L3A21M3+L3A21M8 or Enterococcus faecalis L3B1K3+L3A21M3 | Bacteriocins | Fresh cheese | Listeria monocytogenes | 5 logs (7 d/4°C) | Pros: No negative sensory attributes recorded by non-trained testersCons: Producing strains generally not considered food grade | Coelho et al. (2014) |
| Lactobacillus curvatus BCS35 | Bacteriocins | Young hakeMegrim | ColiformsMesophilesColiformsMesophiles | 0.5–2.2 logs0.4–0.8 logs0.1–0.7 logs0.1–0.7 logs (7 d/0°C –2°C) | Pros: Biopreserved fish worth higher price when evaluated by official fish appraiserListeria spp. not detected in biopreserved batches | Gómez-Sala et al (2016) |
| Lactobacillus curvatus CRL705 | Lactocin 705Lactocin AL705 | Vacuum-packed meat | Brochothrix thermosphactaListeria innocua | ∼3.5 logs∼3 logs (36 d/2°C) | Pros:Bacteriocin producer is as effective as the bacteriocin itself over the 36-day storage periodCons:Low bacteriocin activity from producer in first 2 weeks possibly due to strain adapting to meat environmentInfluence of protective culture on sensory attributes to be confirmed | Castellano and Vignolo (2006) |
| Nisin (25% w/w) | Cellulose films | Minimally processed mangoes | Staphylococcus aureusListeria monocytogenes | 6 logs (6 d/5°C)7 logs (4 d/5°C) | Pros: Antimicrobial films did not interfere with appearance, texture or nutritional values of fruit3% cellulose acetate was more efficient than 9% for producing nisin-incorporated films | Barbosa et al. (2013) |
| Nisin Z (320 AU/ml) and lauric arginate (2% w/v) | Pullulan films | Turkey breastHam slicesRaw beef slices | Salmonella TyphimuriumSalmonella EnteriditisStaphylococcus aureusListeria monocytogenesEscherichia coli | 2.5–4.5 logs/cm23.5–5.1 logs/cm25.53 logs/cm25.62 logs/cm2>4 logs/cm2 (28 d/4°C) | Pros: Additive effect from combinationTargets Gram positives and negatives | Pattanayaiying, H-Kittikun and Cutter (2015) |
| Sakacin A (1.36 AU/mg) | Polyethylene coated paper sheets | Thin cut meat | Listeria monocytogenes | 1.5 logs (48 h/4°C) | Pros:Bacteriocin purification method was rapid involving one-step diafiltration and resultant freeze-dried enriched bacteriocin was free of contaminating proteins | Barbiroli et al. (2017) |
| Packaging material in this study commonly used for food packaging | ||||||
| Nisin (2 g/100g or 6 g/100 g) | Starch/halloysite/nanocomposite films | Soft cheese | Listeria monocytogenes | ∼5 logs (14 d/4°C) | Films containing higher concentration of halloysite nanotubules (6 g/100 g) impeded nisin diffusion and were slightly less effective than those containing 3 g/100 g | Meira et al. (2016) |
| Nisin (106 IU used in sanitiser preparation) | Fresh cut cantaloupe/Rind | Escherichia coli O157:H7SalmonellaListeria monocytogenes | ∼ 2.8CFU/g/3.2 logs/cm2∼2.4 CFU/g/2.9 logs/cm2∼2.3 CFU/g/3.5 logs/cm2(7 d/5°C) | Pros:All compounds in the nisin-based sanitiser have GRAS status granted by the FDA and concentrations used were below recommended dose limit | Ukuko, Huang L and Sommers (2015) | |
| Nisin (500–1000 IU/ml) + ethanol (20%) | Stainless steel | Escherichia coli O157:H7Salmonella | ∼ 5 logs/cm2(15 min) | Pros:Active against Gram negativesCons: Incubation time of 15 min potentially too long for streamlined industrial production | Phongphakdee and Nitisinprasert (2015) | |
| Curvacin A-producing Lactobacillus sakei CRL1862 + curvacin A (266.67 AU/ml) | Stainless steelPolytetrafluoroethylene surfaces (PTFE) | Listeria monocytogenes biofilm | 2.22 logs1.77 logs (6 h) | Pros: Capable of reducing pre-existing biofilmCons: Incubation time required to generate biofilm reduction too long from an industrial perspective | Pérez-Ibarreche et al. (2016) | |
| Enterocin B3A-B3B (0.064 mg/ml) | Stainless steel | Listeria monocytogenes biofilm | 2 logs(24 h) | Pros:Capable of reducing pre-existing biofilm | Al-Seraih et al. (2017) | |
| Nisin (0.256 mg/ml) + enterocin B3A-B3B (0.008 mg/ml) | 1.4 logs (0 h) and 2.3 logs (24 h) | Four-fold less nisin and enterocin required when used in combinationCons :Incubation times potentially too long from an industrial persepective | ||||
| Bacteriocin . | ||||||
|---|---|---|---|---|---|---|
| Bacteriocin (+ additional hurdle) . | Producer . | Food type . | Target microorganism . | Reduction (storage time/temp) . | Pros and cons . | Reference . |
| Lactoccin BZ (1600 AU/ml) | Lactococus lactis | Fresh beef | Listeria innocua | 6 logs (6 d/4–5°C) | Pros: Bacteriocin activity unaffected by meat components or pH of meat | Yildirim et al. (2016) |
| SH01 (1000 AU/g) | Enterococcus faecium | Ground beef | Listeria monocytogenes | 2.33 logs (8 d/20 oC) | Pros: Bacteriocin activity increases with temperatures up to 20oC | Kim, Jung and Kim (2015) |
| Cons: Bacteriocin sensitive to protease activity | ||||||
| Salivaricin KLD (10%) | Lactobacillus salivarius | Creamy filling | Bacillus cereusEnterococcus faecalisPseudomonas stutzeriStaphylococcus hominisStenotrophomonas sp. | 66.9%100%100%25.8%100% (3 d/37°C) | Pros: Bacteriocin stable at high temperature (100°C) and pH 3–10Active against Gram negativesCons: Bacteriocin sensitive to protease activity | Therdtatha et al. (2016) |
| Aureocin A53 (256 AU/ml) | Staphylococcus aureus | Skimmed milk | Listeria monocytogenes | 7.7 logs (7 d/4oC) | Pros: Highly resistant to proteolytic degradation | Fagundes et al. (2016) |
| Cons: Producing strain is a recognised human pathogen and produces two non-classical enterotoxins and a haemolysin | ||||||
| Enterocin AS-48 (250 μg/ml) | Enterococcus faecalis | Sardines (vacuum packed) | Endogenous staphylococci | >1 log (6 d/5°C) | Pros: Decreased levels of biogenic amines cadaverine, putrescine, tyramine and histamine by several fold | Ananou et al. (2014) |
| Cons: Antimicrobial activity against spoilage microbiota not highly effective because most of the spoilage microbiota are Gram negatives | ||||||
| Method of AS-48 application (immersion of | ||||||
| fish in bacteriocin) may be inadequate to ensure sufficiently high bacteriocin concentration on the food | ||||||
| Leukocin K7 (80 AU/ml) + glycine (5 mg/ml) | Leuconostoc mesenteroides | Milk | Listeria monocytogenes | 3 logs (1 d/4 oC) | Pros: Prevented emergence of resistant mutants over 7-day study period | Shi et al. (2016) |
| Nisin +garlic (2.08 mg/g garlic sprout) in phosphptidylcholine nanoliposomes | Lactococcus lactis | Milk | Listeria monocytogenesSalmonella enteriditisEscherichia coliStaphylococcus aureus | ∼6 logs (10 h/37°C)∼3–4 log∼3–4 logs∼5 logs (24 h/37°C) | Pros: Nisin and garlic more effective than either antimicrobial aloneCons: Garlic extract could influence the flavour of the milk. | Pinilla and Brandelli (2016) |
| Enterocin AS-48 (50 μg/g) + high hydrostatic pressure (600 MPa, 8 min) | Enterococcus faecalis A-48-32 | Cherimoya pulp | Artificially contaminated with natural microbiota | >6 logs(1 d/5°C) | Pros: Combined treatment more effective than either treatment alone and reduced Gram positives and Gram negatives.Cons: Microbial counts increased significantly after 15 days of storage | Pérez Pulido et al. (2015) |
| Bacteriocin-producing culture | ||||||
| Culture | Bacteriocin | Food type | Target microorganism | Reduction (storage time/temp) | Pros and cons | Reference |
| Lactobacillus sakei subsp. sakei 2a | Sakacin | Cheese spread | Listeria monocytogenes | ∼3 logs (28 d/15°C) | Pros: Bacteriocin production in cheese during storage was confirmed via expression of bacteriocin genesCons: Impact of bacteriocin producer on cheese flavour to be confirmed | Martinez et al. (2015) |
| Enterococcus faecalis L3A21M3+L3A21M8 or Enterococcus faecalis L3B1K3+L3A21M3 | Bacteriocins | Fresh cheese | Listeria monocytogenes | 5 logs (7 d/4°C) | Pros: No negative sensory attributes recorded by non-trained testersCons: Producing strains generally not considered food grade | Coelho et al. (2014) |
| Lactobacillus curvatus BCS35 | Bacteriocins | Young hakeMegrim | ColiformsMesophilesColiformsMesophiles | 0.5–2.2 logs0.4–0.8 logs0.1–0.7 logs0.1–0.7 logs (7 d/0°C –2°C) | Pros: Biopreserved fish worth higher price when evaluated by official fish appraiserListeria spp. not detected in biopreserved batches | Gómez-Sala et al (2016) |
| Lactobacillus curvatus CRL705 | Lactocin 705Lactocin AL705 | Vacuum-packed meat | Brochothrix thermosphactaListeria innocua | ∼3.5 logs∼3 logs (36 d/2°C) | Pros:Bacteriocin producer is as effective as the bacteriocin itself over the 36-day storage periodCons:Low bacteriocin activity from producer in first 2 weeks possibly due to strain adapting to meat environmentInfluence of protective culture on sensory attributes to be confirmed | Castellano and Vignolo (2006) |
| Nisin (25% w/w) | Cellulose films | Minimally processed mangoes | Staphylococcus aureusListeria monocytogenes | 6 logs (6 d/5°C)7 logs (4 d/5°C) | Pros: Antimicrobial films did not interfere with appearance, texture or nutritional values of fruit3% cellulose acetate was more efficient than 9% for producing nisin-incorporated films | Barbosa et al. (2013) |
| Nisin Z (320 AU/ml) and lauric arginate (2% w/v) | Pullulan films | Turkey breastHam slicesRaw beef slices | Salmonella TyphimuriumSalmonella EnteriditisStaphylococcus aureusListeria monocytogenesEscherichia coli | 2.5–4.5 logs/cm23.5–5.1 logs/cm25.53 logs/cm25.62 logs/cm2>4 logs/cm2 (28 d/4°C) | Pros: Additive effect from combinationTargets Gram positives and negatives | Pattanayaiying, H-Kittikun and Cutter (2015) |
| Sakacin A (1.36 AU/mg) | Polyethylene coated paper sheets | Thin cut meat | Listeria monocytogenes | 1.5 logs (48 h/4°C) | Pros:Bacteriocin purification method was rapid involving one-step diafiltration and resultant freeze-dried enriched bacteriocin was free of contaminating proteins | Barbiroli et al. (2017) |
| Packaging material in this study commonly used for food packaging | ||||||
| Nisin (2 g/100g or 6 g/100 g) | Starch/halloysite/nanocomposite films | Soft cheese | Listeria monocytogenes | ∼5 logs (14 d/4°C) | Films containing higher concentration of halloysite nanotubules (6 g/100 g) impeded nisin diffusion and were slightly less effective than those containing 3 g/100 g | Meira et al. (2016) |
| Nisin (106 IU used in sanitiser preparation) | Fresh cut cantaloupe/Rind | Escherichia coli O157:H7SalmonellaListeria monocytogenes | ∼ 2.8CFU/g/3.2 logs/cm2∼2.4 CFU/g/2.9 logs/cm2∼2.3 CFU/g/3.5 logs/cm2(7 d/5°C) | Pros:All compounds in the nisin-based sanitiser have GRAS status granted by the FDA and concentrations used were below recommended dose limit | Ukuko, Huang L and Sommers (2015) | |
| Nisin (500–1000 IU/ml) + ethanol (20%) | Stainless steel | Escherichia coli O157:H7Salmonella | ∼ 5 logs/cm2(15 min) | Pros:Active against Gram negativesCons: Incubation time of 15 min potentially too long for streamlined industrial production | Phongphakdee and Nitisinprasert (2015) | |
| Curvacin A-producing Lactobacillus sakei CRL1862 + curvacin A (266.67 AU/ml) | Stainless steelPolytetrafluoroethylene surfaces (PTFE) | Listeria monocytogenes biofilm | 2.22 logs1.77 logs (6 h) | Pros: Capable of reducing pre-existing biofilmCons: Incubation time required to generate biofilm reduction too long from an industrial perspective | Pérez-Ibarreche et al. (2016) | |
| Enterocin B3A-B3B (0.064 mg/ml) | Stainless steel | Listeria monocytogenes biofilm | 2 logs(24 h) | Pros:Capable of reducing pre-existing biofilm | Al-Seraih et al. (2017) | |
| Nisin (0.256 mg/ml) + enterocin B3A-B3B (0.008 mg/ml) | 1.4 logs (0 h) and 2.3 logs (24 h) | Four-fold less nisin and enterocin required when used in combinationCons :Incubation times potentially too long from an industrial persepective | ||||
Bacteriocin applications for food safety and quality purposes.
| Bacteriocin . | ||||||
|---|---|---|---|---|---|---|
| Bacteriocin (+ additional hurdle) . | Producer . | Food type . | Target microorganism . | Reduction (storage time/temp) . | Pros and cons . | Reference . |
| Lactoccin BZ (1600 AU/ml) | Lactococus lactis | Fresh beef | Listeria innocua | 6 logs (6 d/4–5°C) | Pros: Bacteriocin activity unaffected by meat components or pH of meat | Yildirim et al. (2016) |
| SH01 (1000 AU/g) | Enterococcus faecium | Ground beef | Listeria monocytogenes | 2.33 logs (8 d/20 oC) | Pros: Bacteriocin activity increases with temperatures up to 20oC | Kim, Jung and Kim (2015) |
| Cons: Bacteriocin sensitive to protease activity | ||||||
| Salivaricin KLD (10%) | Lactobacillus salivarius | Creamy filling | Bacillus cereusEnterococcus faecalisPseudomonas stutzeriStaphylococcus hominisStenotrophomonas sp. | 66.9%100%100%25.8%100% (3 d/37°C) | Pros: Bacteriocin stable at high temperature (100°C) and pH 3–10Active against Gram negativesCons: Bacteriocin sensitive to protease activity | Therdtatha et al. (2016) |
| Aureocin A53 (256 AU/ml) | Staphylococcus aureus | Skimmed milk | Listeria monocytogenes | 7.7 logs (7 d/4oC) | Pros: Highly resistant to proteolytic degradation | Fagundes et al. (2016) |
| Cons: Producing strain is a recognised human pathogen and produces two non-classical enterotoxins and a haemolysin | ||||||
| Enterocin AS-48 (250 μg/ml) | Enterococcus faecalis | Sardines (vacuum packed) | Endogenous staphylococci | >1 log (6 d/5°C) | Pros: Decreased levels of biogenic amines cadaverine, putrescine, tyramine and histamine by several fold | Ananou et al. (2014) |
| Cons: Antimicrobial activity against spoilage microbiota not highly effective because most of the spoilage microbiota are Gram negatives | ||||||
| Method of AS-48 application (immersion of | ||||||
| fish in bacteriocin) may be inadequate to ensure sufficiently high bacteriocin concentration on the food | ||||||
| Leukocin K7 (80 AU/ml) + glycine (5 mg/ml) | Leuconostoc mesenteroides | Milk | Listeria monocytogenes | 3 logs (1 d/4 oC) | Pros: Prevented emergence of resistant mutants over 7-day study period | Shi et al. (2016) |
| Nisin +garlic (2.08 mg/g garlic sprout) in phosphptidylcholine nanoliposomes | Lactococcus lactis | Milk | Listeria monocytogenesSalmonella enteriditisEscherichia coliStaphylococcus aureus | ∼6 logs (10 h/37°C)∼3–4 log∼3–4 logs∼5 logs (24 h/37°C) | Pros: Nisin and garlic more effective than either antimicrobial aloneCons: Garlic extract could influence the flavour of the milk. | Pinilla and Brandelli (2016) |
| Enterocin AS-48 (50 μg/g) + high hydrostatic pressure (600 MPa, 8 min) | Enterococcus faecalis A-48-32 | Cherimoya pulp | Artificially contaminated with natural microbiota | >6 logs(1 d/5°C) | Pros: Combined treatment more effective than either treatment alone and reduced Gram positives and Gram negatives.Cons: Microbial counts increased significantly after 15 days of storage | Pérez Pulido et al. (2015) |
| Bacteriocin-producing culture | ||||||
| Culture | Bacteriocin | Food type | Target microorganism | Reduction (storage time/temp) | Pros and cons | Reference |
| Lactobacillus sakei subsp. sakei 2a | Sakacin | Cheese spread | Listeria monocytogenes | ∼3 logs (28 d/15°C) | Pros: Bacteriocin production in cheese during storage was confirmed via expression of bacteriocin genesCons: Impact of bacteriocin producer on cheese flavour to be confirmed | Martinez et al. (2015) |
| Enterococcus faecalis L3A21M3+L3A21M8 or Enterococcus faecalis L3B1K3+L3A21M3 | Bacteriocins | Fresh cheese | Listeria monocytogenes | 5 logs (7 d/4°C) | Pros: No negative sensory attributes recorded by non-trained testersCons: Producing strains generally not considered food grade | Coelho et al. (2014) |
| Lactobacillus curvatus BCS35 | Bacteriocins | Young hakeMegrim | ColiformsMesophilesColiformsMesophiles | 0.5–2.2 logs0.4–0.8 logs0.1–0.7 logs0.1–0.7 logs (7 d/0°C –2°C) | Pros: Biopreserved fish worth higher price when evaluated by official fish appraiserListeria spp. not detected in biopreserved batches | Gómez-Sala et al (2016) |
| Lactobacillus curvatus CRL705 | Lactocin 705Lactocin AL705 | Vacuum-packed meat | Brochothrix thermosphactaListeria innocua | ∼3.5 logs∼3 logs (36 d/2°C) | Pros:Bacteriocin producer is as effective as the bacteriocin itself over the 36-day storage periodCons:Low bacteriocin activity from producer in first 2 weeks possibly due to strain adapting to meat environmentInfluence of protective culture on sensory attributes to be confirmed | Castellano and Vignolo (2006) |
| Nisin (25% w/w) | Cellulose films | Minimally processed mangoes | Staphylococcus aureusListeria monocytogenes | 6 logs (6 d/5°C)7 logs (4 d/5°C) | Pros: Antimicrobial films did not interfere with appearance, texture or nutritional values of fruit3% cellulose acetate was more efficient than 9% for producing nisin-incorporated films | Barbosa et al. (2013) |
| Nisin Z (320 AU/ml) and lauric arginate (2% w/v) | Pullulan films | Turkey breastHam slicesRaw beef slices | Salmonella TyphimuriumSalmonella EnteriditisStaphylococcus aureusListeria monocytogenesEscherichia coli | 2.5–4.5 logs/cm23.5–5.1 logs/cm25.53 logs/cm25.62 logs/cm2>4 logs/cm2 (28 d/4°C) | Pros: Additive effect from combinationTargets Gram positives and negatives | Pattanayaiying, H-Kittikun and Cutter (2015) |
| Sakacin A (1.36 AU/mg) | Polyethylene coated paper sheets | Thin cut meat | Listeria monocytogenes | 1.5 logs (48 h/4°C) | Pros:Bacteriocin purification method was rapid involving one-step diafiltration and resultant freeze-dried enriched bacteriocin was free of contaminating proteins | Barbiroli et al. (2017) |
| Packaging material in this study commonly used for food packaging | ||||||
| Nisin (2 g/100g or 6 g/100 g) | Starch/halloysite/nanocomposite films | Soft cheese | Listeria monocytogenes | ∼5 logs (14 d/4°C) | Films containing higher concentration of halloysite nanotubules (6 g/100 g) impeded nisin diffusion and were slightly less effective than those containing 3 g/100 g | Meira et al. (2016) |
| Nisin (106 IU used in sanitiser preparation) | Fresh cut cantaloupe/Rind | Escherichia coli O157:H7SalmonellaListeria monocytogenes | ∼ 2.8CFU/g/3.2 logs/cm2∼2.4 CFU/g/2.9 logs/cm2∼2.3 CFU/g/3.5 logs/cm2(7 d/5°C) | Pros:All compounds in the nisin-based sanitiser have GRAS status granted by the FDA and concentrations used were below recommended dose limit | Ukuko, Huang L and Sommers (2015) | |
| Nisin (500–1000 IU/ml) + ethanol (20%) | Stainless steel | Escherichia coli O157:H7Salmonella | ∼ 5 logs/cm2(15 min) | Pros:Active against Gram negativesCons: Incubation time of 15 min potentially too long for streamlined industrial production | Phongphakdee and Nitisinprasert (2015) | |
| Curvacin A-producing Lactobacillus sakei CRL1862 + curvacin A (266.67 AU/ml) | Stainless steelPolytetrafluoroethylene surfaces (PTFE) | Listeria monocytogenes biofilm | 2.22 logs1.77 logs (6 h) | Pros: Capable of reducing pre-existing biofilmCons: Incubation time required to generate biofilm reduction too long from an industrial perspective | Pérez-Ibarreche et al. (2016) | |
| Enterocin B3A-B3B (0.064 mg/ml) | Stainless steel | Listeria monocytogenes biofilm | 2 logs(24 h) | Pros:Capable of reducing pre-existing biofilm | Al-Seraih et al. (2017) | |
| Nisin (0.256 mg/ml) + enterocin B3A-B3B (0.008 mg/ml) | 1.4 logs (0 h) and 2.3 logs (24 h) | Four-fold less nisin and enterocin required when used in combinationCons :Incubation times potentially too long from an industrial persepective | ||||
| Bacteriocin . | ||||||
|---|---|---|---|---|---|---|
| Bacteriocin (+ additional hurdle) . | Producer . | Food type . | Target microorganism . | Reduction (storage time/temp) . | Pros and cons . | Reference . |
| Lactoccin BZ (1600 AU/ml) | Lactococus lactis | Fresh beef | Listeria innocua | 6 logs (6 d/4–5°C) | Pros: Bacteriocin activity unaffected by meat components or pH of meat | Yildirim et al. (2016) |
| SH01 (1000 AU/g) | Enterococcus faecium | Ground beef | Listeria monocytogenes | 2.33 logs (8 d/20 oC) | Pros: Bacteriocin activity increases with temperatures up to 20oC | Kim, Jung and Kim (2015) |
| Cons: Bacteriocin sensitive to protease activity | ||||||
| Salivaricin KLD (10%) | Lactobacillus salivarius | Creamy filling | Bacillus cereusEnterococcus faecalisPseudomonas stutzeriStaphylococcus hominisStenotrophomonas sp. | 66.9%100%100%25.8%100% (3 d/37°C) | Pros: Bacteriocin stable at high temperature (100°C) and pH 3–10Active against Gram negativesCons: Bacteriocin sensitive to protease activity | Therdtatha et al. (2016) |
| Aureocin A53 (256 AU/ml) | Staphylococcus aureus | Skimmed milk | Listeria monocytogenes | 7.7 logs (7 d/4oC) | Pros: Highly resistant to proteolytic degradation | Fagundes et al. (2016) |
| Cons: Producing strain is a recognised human pathogen and produces two non-classical enterotoxins and a haemolysin | ||||||
| Enterocin AS-48 (250 μg/ml) | Enterococcus faecalis | Sardines (vacuum packed) | Endogenous staphylococci | >1 log (6 d/5°C) | Pros: Decreased levels of biogenic amines cadaverine, putrescine, tyramine and histamine by several fold | Ananou et al. (2014) |
| Cons: Antimicrobial activity against spoilage microbiota not highly effective because most of the spoilage microbiota are Gram negatives | ||||||
| Method of AS-48 application (immersion of | ||||||
| fish in bacteriocin) may be inadequate to ensure sufficiently high bacteriocin concentration on the food | ||||||
| Leukocin K7 (80 AU/ml) + glycine (5 mg/ml) | Leuconostoc mesenteroides | Milk | Listeria monocytogenes | 3 logs (1 d/4 oC) | Pros: Prevented emergence of resistant mutants over 7-day study period | Shi et al. (2016) |
| Nisin +garlic (2.08 mg/g garlic sprout) in phosphptidylcholine nanoliposomes | Lactococcus lactis | Milk | Listeria monocytogenesSalmonella enteriditisEscherichia coliStaphylococcus aureus | ∼6 logs (10 h/37°C)∼3–4 log∼3–4 logs∼5 logs (24 h/37°C) | Pros: Nisin and garlic more effective than either antimicrobial aloneCons: Garlic extract could influence the flavour of the milk. | Pinilla and Brandelli (2016) |
| Enterocin AS-48 (50 μg/g) + high hydrostatic pressure (600 MPa, 8 min) | Enterococcus faecalis A-48-32 | Cherimoya pulp | Artificially contaminated with natural microbiota | >6 logs(1 d/5°C) | Pros: Combined treatment more effective than either treatment alone and reduced Gram positives and Gram negatives.Cons: Microbial counts increased significantly after 15 days of storage | Pérez Pulido et al. (2015) |
| Bacteriocin-producing culture | ||||||
| Culture | Bacteriocin | Food type | Target microorganism | Reduction (storage time/temp) | Pros and cons | Reference |
| Lactobacillus sakei subsp. sakei 2a | Sakacin | Cheese spread | Listeria monocytogenes | ∼3 logs (28 d/15°C) | Pros: Bacteriocin production in cheese during storage was confirmed via expression of bacteriocin genesCons: Impact of bacteriocin producer on cheese flavour to be confirmed | Martinez et al. (2015) |
| Enterococcus faecalis L3A21M3+L3A21M8 or Enterococcus faecalis L3B1K3+L3A21M3 | Bacteriocins | Fresh cheese | Listeria monocytogenes | 5 logs (7 d/4°C) | Pros: No negative sensory attributes recorded by non-trained testersCons: Producing strains generally not considered food grade | Coelho et al. (2014) |
| Lactobacillus curvatus BCS35 | Bacteriocins | Young hakeMegrim | ColiformsMesophilesColiformsMesophiles | 0.5–2.2 logs0.4–0.8 logs0.1–0.7 logs0.1–0.7 logs (7 d/0°C –2°C) | Pros: Biopreserved fish worth higher price when evaluated by official fish appraiserListeria spp. not detected in biopreserved batches | Gómez-Sala et al (2016) |
| Lactobacillus curvatus CRL705 | Lactocin 705Lactocin AL705 | Vacuum-packed meat | Brochothrix thermosphactaListeria innocua | ∼3.5 logs∼3 logs (36 d/2°C) | Pros:Bacteriocin producer is as effective as the bacteriocin itself over the 36-day storage periodCons:Low bacteriocin activity from producer in first 2 weeks possibly due to strain adapting to meat environmentInfluence of protective culture on sensory attributes to be confirmed | Castellano and Vignolo (2006) |
| Nisin (25% w/w) | Cellulose films | Minimally processed mangoes | Staphylococcus aureusListeria monocytogenes | 6 logs (6 d/5°C)7 logs (4 d/5°C) | Pros: Antimicrobial films did not interfere with appearance, texture or nutritional values of fruit3% cellulose acetate was more efficient than 9% for producing nisin-incorporated films | Barbosa et al. (2013) |
| Nisin Z (320 AU/ml) and lauric arginate (2% w/v) | Pullulan films | Turkey breastHam slicesRaw beef slices | Salmonella TyphimuriumSalmonella EnteriditisStaphylococcus aureusListeria monocytogenesEscherichia coli | 2.5–4.5 logs/cm23.5–5.1 logs/cm25.53 logs/cm25.62 logs/cm2>4 logs/cm2 (28 d/4°C) | Pros: Additive effect from combinationTargets Gram positives and negatives | Pattanayaiying, H-Kittikun and Cutter (2015) |
| Sakacin A (1.36 AU/mg) | Polyethylene coated paper sheets | Thin cut meat | Listeria monocytogenes | 1.5 logs (48 h/4°C) | Pros:Bacteriocin purification method was rapid involving one-step diafiltration and resultant freeze-dried enriched bacteriocin was free of contaminating proteins | Barbiroli et al. (2017) |
| Packaging material in this study commonly used for food packaging | ||||||
| Nisin (2 g/100g or 6 g/100 g) | Starch/halloysite/nanocomposite films | Soft cheese | Listeria monocytogenes | ∼5 logs (14 d/4°C) | Films containing higher concentration of halloysite nanotubules (6 g/100 g) impeded nisin diffusion and were slightly less effective than those containing 3 g/100 g | Meira et al. (2016) |
| Nisin (106 IU used in sanitiser preparation) | Fresh cut cantaloupe/Rind | Escherichia coli O157:H7SalmonellaListeria monocytogenes | ∼ 2.8CFU/g/3.2 logs/cm2∼2.4 CFU/g/2.9 logs/cm2∼2.3 CFU/g/3.5 logs/cm2(7 d/5°C) | Pros:All compounds in the nisin-based sanitiser have GRAS status granted by the FDA and concentrations used were below recommended dose limit | Ukuko, Huang L and Sommers (2015) | |
| Nisin (500–1000 IU/ml) + ethanol (20%) | Stainless steel | Escherichia coli O157:H7Salmonella | ∼ 5 logs/cm2(15 min) | Pros:Active against Gram negativesCons: Incubation time of 15 min potentially too long for streamlined industrial production | Phongphakdee and Nitisinprasert (2015) | |
| Curvacin A-producing Lactobacillus sakei CRL1862 + curvacin A (266.67 AU/ml) | Stainless steelPolytetrafluoroethylene surfaces (PTFE) | Listeria monocytogenes biofilm | 2.22 logs1.77 logs (6 h) | Pros: Capable of reducing pre-existing biofilmCons: Incubation time required to generate biofilm reduction too long from an industrial perspective | Pérez-Ibarreche et al. (2016) | |
| Enterocin B3A-B3B (0.064 mg/ml) | Stainless steel | Listeria monocytogenes biofilm | 2 logs(24 h) | Pros:Capable of reducing pre-existing biofilm | Al-Seraih et al. (2017) | |
| Nisin (0.256 mg/ml) + enterocin B3A-B3B (0.008 mg/ml) | 1.4 logs (0 h) and 2.3 logs (24 h) | Four-fold less nisin and enterocin required when used in combinationCons :Incubation times potentially too long from an industrial persepective | ||||
Direct addition of pure or partially purified bacteriocin to the food product
Bacteriocins can be incorporated into the food matrix or can be applied to the surface of the food but the method of application is most often dictated by the food type. However, bacteriocin efficacy in the food environment can be influenced by a number of factors (Schillinger, Geisen and Holzapfel 1996) including environmental pH, its solubility and distribution in the food matrix, binding of the bacteriocin to food components including fat and protein, inactivation by other substances such as additives, susceptibility to proteases and oxidation processes and the emergence of resistant mutants. Innovations such as the use of encapsulation technologies to protect and ensure controlled release of antimicrobial peptides as well as antimicrobial packaging should help to overcome some of these issues. In this regard, it is essential to test bacteriocin efficacy in the intended food environment.
For example, the class IIa bacteriocin plantaricin BM-1 proved more effective at inhibiting L. monocytogenes growth during storage at 4°C for 35 days in cooked ham (without any chemical preservatives) when applied to the surface of one side of each ham slice than when incorporated internally into the ham just prior to homogenisation of the meat paste (Zhou et al.2015). The surface applied bacteriocin treatment (1280 AU/g) reduced Listeria counts below the detection limit on the first day of storage whereas the incorporated bacteriocin (at the same concentration) failed to decrease the initial inoculum. The authors suggest that the incorporated bacteriocin lost effectiveness owing to a higher adsorption of bacteriocin molecules to meat components, slower diffusion and uneven distribution of the bacteriocin in the food matrix as well as exposure to heat treatment and mechanical stirring which presumably impacted bacteriocin activity.
Nanoencapsulation of bacteriocins is gaining attention owing to the fact that the encapsulated bacteriocin is protected from degradation by proteases and interactions with food components, and in some instances the encapsulated bacteriocin exhibits even greater antimicrobial activity (da Silva Malheiros et al.2012; Prombutara et al.2012; Thirumurugan, Ramachandran and Gowri 2013). Nanodelivery systems can be lipid, carbohydrate, metal or polymer based (Fahim, Khairalla and El-Gendy 2016) and can enable the controlled release of bacteriocin into the food. For example, nisin encapsulated in dipalmitoylphosphatidylcholine liposomes remained active in raw ground beef whereas unencapsulated nisin activity could not be detected 30 min after its addition (Boualem et al.2013). At temperatures above 37°C, the liposomes melted ensuring the controlled release of nisin into the food matrix.
Martinez et al. (2016) assessed the inhibitory activity of free and encapsulated nisin (from Nisaplin®) against L. monocytogenes and the spore former Bacillus cereus. Interestingly, many LAB bacteriocins exhibit sporicidal/sporostatic activity (recently reviewed by Egan et al.2016). The encapsulating agent used in the study was gum Arabic, a natural resin composed of glycoproteins and polysaccharides, which is odourless, tasteless and non-toxic, and the method used for encapsulation involved spray drying. A combination of free and encapsulated nisin (0.5 mg/L each) exhibited the most effective antilisterial activity in refrigerated skimmed and whole milk over 21 days. However, the authors confirm that the emergence of nisin-resistant subpopulations of Listeria suggests that the encapsulation method intended for delayed bacteriocin release needs to be improved. Encapsulated nisin (0.25 mg/L) proved as effective as free nisin (at the same concentration) for inhibiting B. cereus spore germination and the outgrowth of vegetative cells where both cells and spores were undetectable at day 21 in skimmed and whole milk.
Indeed, bacteriocin resistance is a serious concern and a complex phenomenon generally involving the bacterial cell envelope and arises at frequencies of between 10−9 and 10−2, depending on the class of bacteriocin (Bastos, Coelho and Santos 2015). Successful strategies to prevent the development of bacteriocin resistance include the exploitation of bacteriocins as part of multihurdle approaches (Bastos, Coelho and Santos 2015). Such approaches can result in additive or synergistic effects, reducing the required concentrations of both antimicrobials and as mentioned already can even expand the killing spectrum of bacteriocins to include Gram negatives (Prudêncio, dos Santos and Dantas Vanetti 2015). Common antimicrobials which have been paired with bacteriocins include organic acids (Grande et al.2006), chelating agents (Martin-Visscher et al.2011), other bacteriocins (Kaur, Singh and Malik 2013) and essential oils (Turgis et al.2012) as well as processes such as high-pressure processing (Pérez Pulido et al.2015), pulsed electric field (Martínez Viedma et al. 2009) and temperature (Phillips and Duggan 2002) (Table 1).
Macwana and Muriana (2012) developed a methodical approach for identifying best bacteriocin mixtures for use in food preservation based on mechanism of resistance and revealed that mixtures of bacteriocins with different modes of action provided greater inhibition than using mixtures of bacteriocins from the same classes.
Guardian (Gillco Ingredients, San Marcos, CA, USA) is an example of a commercially available multihurdle antimicrobial ingredient based on the synergy between nisin and rosemary (http://www.gillco.com/pr_antim-novagard.php) and can be used in to replace chemical preservatives. It targets Gram-positive pathogens by killing and/or delaying their growth and the rosemary also helps to reduce fat oxidation. Its main applications include soups, sauces, cooked sausages, salad dressings and deli salads.
In general, the direct of addition of purified bacteriocins to food is likely to be a less appealing route for the food manufacturer since bacteriocin purification is a costly process where it has been estimated that 30% of the total production cost is due to the complex nutritional media required for growth of the fastidious LAB producers (Bali, Panesar and Bera 2016). Cost-effective production of bacteriocins is currently an active area of research (Bali, Panesar and Bera 2016) and should ensure that the use of pure bacteriocins is a more viable option in the future. Partially purified bacteriocins, such as ALTA 2351, produced using food-grade substrates such as milk or whey is an alternative and cheaper option. However, both strategies require specific approval from a legal standpoint for use as food preservatives (Vignolo et al.2012).
Bacteriocin-producing cultures
A bacteriocin-producing culture is generally a cheaper option for food safety and biopreservation strategies since it does not require bacteriocin isolation and purification and there are fewer legal restrictions. The bacteriocin producer may also serve as the starter culture in fermented foods. Interestingly, certain strains can produce more than one bacteriocin, for example, L. lactis LMG2081 was recently shown to produce a novel lantibiotic, lacticin LMG, as well as the class IIb bacteriocin lactococcin G; thus, protective cultures can be multibacteriocinogenic (Mirkovic et al.2016). If using an additional safety/protective culture it is essential that it does not interfere with the organoleptic properties of the food or the activity of the starter culture. Likewise, bacteriocin-producing strains must be compatible with other microorganisms in the food system which must also provide a suitable environment for bacteriocin production (Schillinger, Geisen and Holzapfel 1996). Other risk factors include the possibility of phage infection or the spontaneous loss of bacteriocin producing ability (Schillinger, Geisen and Holzapfel 1996). The latter is a particular risk for bacteriocins encoded on extrachromosomal elements such as plasmids. However, in situ bacteriocin production is a viable strategy and commercially available bacteriocin-producing safety cultures include the nisin-producing DairySafe range (CSK Food Enrichment, The Netherlands), the nisin-producing BioSafe range (Chr. Hansen, Denmark) both for dairy applications, and Bactoferm-LC (Chr Hansen) a freeze-dried culture blend consisting of P. acidilactici and Lactobacillus curvatus which is capable of acidification and produces the class II bacteriocins pediocin PA1 and sakacin A, and is recommended for control of L. monocytogenes in meat products (Ghrairi, Chaftar and Jani 2012). Research continues to report on the efficacy of new bacteriocin-producing cultures in food systems (Table 1), and in some instances the bacteriocin-producing culture has been proven to be as effective as using the bacteriocin itself.
Antimicrobial packaging
The use of bacteriocins in antimicrobial packaging is particularly suited for foods at risk of surface contamination (Table 1). In this regard, the bacteriocin is afforded protection from interaction with food components, thus reducing the risk of bacteriocin inactivation, and its release onto the food surface can be controlled. The bacteriocins can be either incorporated directly into the film matrix or coated onto the surface of the film (Woraprayote et al.2016). However, as stated by O’ Connor et al. (2015) it is necessary to understand the mode of action of bacteriocins for use in such applications along with their physico-chemical properties. As an example, nisin proved more bioactive against food pathogens when absorbed onto hydrophilic surfaces which absorbed higher quantities of the bacteriocin compared to hydrophobic surfaces (Karam et al.2013).
The results to date clearly suggest that antimicrobial packaging is a promising means of limiting bacterial growth on foods through the use of bacteriocins (Table 1) (Barbosa et al.2013; Pattanayaiying, H-Kittikun and Cutter 2015; Meira et al.2016; Barbiroli et al.2017). However, it is important to note that within the European Union only authorised additives can be used in such applications (EU 2009). The legislation in the USA states that ‘the overall regulatory status of a food contact material is dictated by the regulatory status of each individual substance’ in the material (FDA 2015); thus, it is the manufacturer's responsibility to ensure that all components comply with the requirements of the act.
Bacteriocin sanitisers
Sanitisers can be used to reduce the microbial load on food surfaces and equipment or on the food itself (Table 1). However, bacteria growing on food surfaces and equipment often form biofilms where they are surrounded by a matrix of exopolymeric substances (polysaccharides, proteins, DNA and lipids) and can be difficult to remove (Coughlan et al.2016). Several bacteriocins have been shown to restrict biofilm formation or reduce pre-existing biofilms, e.g. nisin (García-Almendárez et al.2008), sakacin 1 (Winkelströter et al.2011), sonorensin (Chopra et al.2015) and plantaricin (Winkelströter, Tulini and De Martinis 2015) (reviewed by Coughlan et al.2016). Interestingly, a bioengineered nisin derivative, termed M21A, was recently shown to be significantly more effective at eradicating an established L. monocytogenes biofilm when used alone (0.1 μg/ml) or in combination with citric acid (175 μg/ml) or cinnamaldehyde (35 μg/ml) than the natural variant, nisin A (Smith et al.2016).
A potential limitation associated with the use bacteriocin sanitisers for industrial applications is the time required to generate meaningful reductions in bacterial numbers. For example, a combination of the class IIb bacteriocin enterocin B3A-B3B with nisin generated a 2 log reduction in L. monocytogenes biofilm on stainless steel surface in 24 h (Al-Seraih et al.2017). In contrast, nisin combined with ethanol was capable of reducing Escherichia coli and Salmonella numbers on stainless steel by 5 logs within 15 min (Phongphakdee and Nitisinprasert 2015). The successful use of bacteriocins as sanitisers will most likely benefit from strategic combinations with other antimicrobials ensuring rapid reductions in bacterial numbers and eliminating the risk of bacteriocin-resistant mutants.
Bacteriophage
Phage can be used at various stages of the food chain from agricultural production right through to food packaging, with special considerations for each stage to ensure optimal efficacy. In this regard, phage applications for food biocontrol can be grouped as follows (Table 2, Fig. 3): (1) post-harvest applications which include (i) direct application of phage to food; (ii) phage-containing antimicrobial packaging; (iii) biosanitation (for food equipment and surfaces) and (2) pre-harvest applications (animals and plants during growth).
Phage applications for food safety purposes.
| Post-harvest . | ||||
|---|---|---|---|---|
| Direct application of phage . | ||||
| Bacteriophage . | Food type . | Target microorganism . | Reduction (storage time/temp) . | Reference . |
| ListShield (106–108 PFU/ml) | Lettuce | Listeria monocytogenes | 91% (5 min) | Perera et al. (2015) |
| Cheese | 82% (5 min) | |||
| Smoked Salmon | 90% (24 h/4°C) | |||
| Frozen entrèes | 99% (24 h) | |||
| Apple slices | 93% (24 h/4°C) | |||
| Phage OSY-SP | Cut green pepper | Escherichia coli O157:H7 | 2.4–3 log CFU/g (3 d/4°C or 4 h/25°C+4°C/3 d) | Snyder, Perry and Yousef (2016) |
| Spinach leaves | 3.4–3.5 log CFU/g (3 d/4°C or 4 h/25°C+4°C/3 d) | |||
| Group II virulent phage (108 PFU/ml) | Chicken liver (homogenised) | Campylobacter jejuni | 0.2–0.7 log CFU/g (48 h/4°C) | Firlieyanti, Connerton and Connerton (2016) |
| SalmoFresh (107 PFU/ml) | Turkey breast cutlets | Salmonella enterica serotype Heidleberg | 1.3 log CFU/g (7 d/4°C) | Sharma, Dhakal and Nannapaneni (2015) |
| Phage FWLLm1 (MOI = 100) | Milk | Listeria monocytogenes | Below detection limit (10 d/4°C) | Rodríguez-Rubio et al. (2015) |
| + Coagulin C23 (584 AU/ml) | ||||
| P100 (2.3 × 107 PFU/ml) | Salmon | Listeria monocytogenes | Below detection limit (1 d/4°C) | Baños et al. (2016) |
| + Enterocin AS-48 (0.37 μg) | Hake | Below detection limit (2 d/4°C) | ||
| Phage-containing antimicrobial packaging | ||||
| Bacteriophage + type of Packaging | Food type | Target microorganism | Reduction (storage time/temp) | Reference |
| Escherichia coli phage + modified cellulose membranes | Cooked turkey breast | Escherichia coli O157:H7 | (All 15 d/4°C) | Anany et al. (2011) |
| Aerobic storage | ∼1.2 log CFU/g | |||
| Modified atmospheric | ∼2 log CFU/g | |||
| Packaging | ||||
| Vacuum | >4 log CFU/g | |||
| Escherichia coli phage + paper coated with encapsulated phage or impregnated with phage suspension | Alfalfa seeds Alfalfa sprouts | Escherichia coli O104:H4 | Below detection limit(1 h)1 log (5 d/RT) | Lone et al. (2016) |
| LISTEXP100 + immobilised on modified cellulose membranes | Cooked turkey | Listeria monocytogenes | >1 log CFU/cm2(25 d/4°C) | Lone et al. (2016) |
| P22 (108 PFU/ml) | Stainless steel | Salmonella Typhimurium biofilm formation | >90% (24 h) | Karaca, Akcelik and Akcelik (2015) |
| ∼90% (48 h) | ||||
| ∼85% (72 h) | ||||
| Salmonella Typhimurium preformed biofilm: 72 h48 h24 h | <10%∼35%>65% | |||
| Phage cocktail (108 PFU/ml) | Stainless steel Plastic | Hydrogen sulphide-producing bacteria-free cells | 2.3 log CFU/cm2 2.7 log CFU/cm2 | Gong and Jiang (2015) |
| Stainless steel Plastic | Hydrogen sulphide-producing bacteria biofilms | 2 log CFU/cm2 1.5 log CFU/cm2 (6 h/30°C) | ||
| LISTEXP100 | Stainless steel wafers | Listeria monocytogenes biofilm | Complete elimination (24 h/20°C) | Iacumin, Manzano and Comi (2016) |
| (108 PFU/ml) | ||||
| Pre-harvest | ||||
| Phage | Animal/crop | Target microorganism | Reduction | Reference |
| UAB_Phi20, UAB_Phi78, UAB_Phi87 encapsulated in liposomes (1011 PFU/ml) | Broiler chickens | Salmonella | ∼4 log CFU/g (6 d post-infection) | Colom et al. (2015) |
| CEV1, CEV2(1011 PFU/ml) | Sheep | Resident Escherichia coli O157:H7 | 99.9% | Raya et al. (2011) |
| CP14 (Group III) | Broiler chickens | Campylobacter jejuni | >3 log CFU/ml | Hammer et al. (2014) |
| CP68 (Group II) | ||||
| Myoviridae | Potato | Dickeya dadantii | No disease progression | Soleimani-Delfan et al. (2015) |
| Siphoviridae | ||||
| Post-harvest . | ||||
|---|---|---|---|---|
| Direct application of phage . | ||||
| Bacteriophage . | Food type . | Target microorganism . | Reduction (storage time/temp) . | Reference . |
| ListShield (106–108 PFU/ml) | Lettuce | Listeria monocytogenes | 91% (5 min) | Perera et al. (2015) |
| Cheese | 82% (5 min) | |||
| Smoked Salmon | 90% (24 h/4°C) | |||
| Frozen entrèes | 99% (24 h) | |||
| Apple slices | 93% (24 h/4°C) | |||
| Phage OSY-SP | Cut green pepper | Escherichia coli O157:H7 | 2.4–3 log CFU/g (3 d/4°C or 4 h/25°C+4°C/3 d) | Snyder, Perry and Yousef (2016) |
| Spinach leaves | 3.4–3.5 log CFU/g (3 d/4°C or 4 h/25°C+4°C/3 d) | |||
| Group II virulent phage (108 PFU/ml) | Chicken liver (homogenised) | Campylobacter jejuni | 0.2–0.7 log CFU/g (48 h/4°C) | Firlieyanti, Connerton and Connerton (2016) |
| SalmoFresh (107 PFU/ml) | Turkey breast cutlets | Salmonella enterica serotype Heidleberg | 1.3 log CFU/g (7 d/4°C) | Sharma, Dhakal and Nannapaneni (2015) |
| Phage FWLLm1 (MOI = 100) | Milk | Listeria monocytogenes | Below detection limit (10 d/4°C) | Rodríguez-Rubio et al. (2015) |
| + Coagulin C23 (584 AU/ml) | ||||
| P100 (2.3 × 107 PFU/ml) | Salmon | Listeria monocytogenes | Below detection limit (1 d/4°C) | Baños et al. (2016) |
| + Enterocin AS-48 (0.37 μg) | Hake | Below detection limit (2 d/4°C) | ||
| Phage-containing antimicrobial packaging | ||||
| Bacteriophage + type of Packaging | Food type | Target microorganism | Reduction (storage time/temp) | Reference |
| Escherichia coli phage + modified cellulose membranes | Cooked turkey breast | Escherichia coli O157:H7 | (All 15 d/4°C) | Anany et al. (2011) |
| Aerobic storage | ∼1.2 log CFU/g | |||
| Modified atmospheric | ∼2 log CFU/g | |||
| Packaging | ||||
| Vacuum | >4 log CFU/g | |||
| Escherichia coli phage + paper coated with encapsulated phage or impregnated with phage suspension | Alfalfa seeds Alfalfa sprouts | Escherichia coli O104:H4 | Below detection limit(1 h)1 log (5 d/RT) | Lone et al. (2016) |
| LISTEXP100 + immobilised on modified cellulose membranes | Cooked turkey | Listeria monocytogenes | >1 log CFU/cm2(25 d/4°C) | Lone et al. (2016) |
| P22 (108 PFU/ml) | Stainless steel | Salmonella Typhimurium biofilm formation | >90% (24 h) | Karaca, Akcelik and Akcelik (2015) |
| ∼90% (48 h) | ||||
| ∼85% (72 h) | ||||
| Salmonella Typhimurium preformed biofilm: 72 h48 h24 h | <10%∼35%>65% | |||
| Phage cocktail (108 PFU/ml) | Stainless steel Plastic | Hydrogen sulphide-producing bacteria-free cells | 2.3 log CFU/cm2 2.7 log CFU/cm2 | Gong and Jiang (2015) |
| Stainless steel Plastic | Hydrogen sulphide-producing bacteria biofilms | 2 log CFU/cm2 1.5 log CFU/cm2 (6 h/30°C) | ||
| LISTEXP100 | Stainless steel wafers | Listeria monocytogenes biofilm | Complete elimination (24 h/20°C) | Iacumin, Manzano and Comi (2016) |
| (108 PFU/ml) | ||||
| Pre-harvest | ||||
| Phage | Animal/crop | Target microorganism | Reduction | Reference |
| UAB_Phi20, UAB_Phi78, UAB_Phi87 encapsulated in liposomes (1011 PFU/ml) | Broiler chickens | Salmonella | ∼4 log CFU/g (6 d post-infection) | Colom et al. (2015) |
| CEV1, CEV2(1011 PFU/ml) | Sheep | Resident Escherichia coli O157:H7 | 99.9% | Raya et al. (2011) |
| CP14 (Group III) | Broiler chickens | Campylobacter jejuni | >3 log CFU/ml | Hammer et al. (2014) |
| CP68 (Group II) | ||||
| Myoviridae | Potato | Dickeya dadantii | No disease progression | Soleimani-Delfan et al. (2015) |
| Siphoviridae | ||||
Phage applications for food safety purposes.
| Post-harvest . | ||||
|---|---|---|---|---|
| Direct application of phage . | ||||
| Bacteriophage . | Food type . | Target microorganism . | Reduction (storage time/temp) . | Reference . |
| ListShield (106–108 PFU/ml) | Lettuce | Listeria monocytogenes | 91% (5 min) | Perera et al. (2015) |
| Cheese | 82% (5 min) | |||
| Smoked Salmon | 90% (24 h/4°C) | |||
| Frozen entrèes | 99% (24 h) | |||
| Apple slices | 93% (24 h/4°C) | |||
| Phage OSY-SP | Cut green pepper | Escherichia coli O157:H7 | 2.4–3 log CFU/g (3 d/4°C or 4 h/25°C+4°C/3 d) | Snyder, Perry and Yousef (2016) |
| Spinach leaves | 3.4–3.5 log CFU/g (3 d/4°C or 4 h/25°C+4°C/3 d) | |||
| Group II virulent phage (108 PFU/ml) | Chicken liver (homogenised) | Campylobacter jejuni | 0.2–0.7 log CFU/g (48 h/4°C) | Firlieyanti, Connerton and Connerton (2016) |
| SalmoFresh (107 PFU/ml) | Turkey breast cutlets | Salmonella enterica serotype Heidleberg | 1.3 log CFU/g (7 d/4°C) | Sharma, Dhakal and Nannapaneni (2015) |
| Phage FWLLm1 (MOI = 100) | Milk | Listeria monocytogenes | Below detection limit (10 d/4°C) | Rodríguez-Rubio et al. (2015) |
| + Coagulin C23 (584 AU/ml) | ||||
| P100 (2.3 × 107 PFU/ml) | Salmon | Listeria monocytogenes | Below detection limit (1 d/4°C) | Baños et al. (2016) |
| + Enterocin AS-48 (0.37 μg) | Hake | Below detection limit (2 d/4°C) | ||
| Phage-containing antimicrobial packaging | ||||
| Bacteriophage + type of Packaging | Food type | Target microorganism | Reduction (storage time/temp) | Reference |
| Escherichia coli phage + modified cellulose membranes | Cooked turkey breast | Escherichia coli O157:H7 | (All 15 d/4°C) | Anany et al. (2011) |
| Aerobic storage | ∼1.2 log CFU/g | |||
| Modified atmospheric | ∼2 log CFU/g | |||
| Packaging | ||||
| Vacuum | >4 log CFU/g | |||
| Escherichia coli phage + paper coated with encapsulated phage or impregnated with phage suspension | Alfalfa seeds Alfalfa sprouts | Escherichia coli O104:H4 | Below detection limit(1 h)1 log (5 d/RT) | Lone et al. (2016) |
| LISTEXP100 + immobilised on modified cellulose membranes | Cooked turkey | Listeria monocytogenes | >1 log CFU/cm2(25 d/4°C) | Lone et al. (2016) |
| P22 (108 PFU/ml) | Stainless steel | Salmonella Typhimurium biofilm formation | >90% (24 h) | Karaca, Akcelik and Akcelik (2015) |
| ∼90% (48 h) | ||||
| ∼85% (72 h) | ||||
| Salmonella Typhimurium preformed biofilm: 72 h48 h24 h | <10%∼35%>65% | |||
| Phage cocktail (108 PFU/ml) | Stainless steel Plastic | Hydrogen sulphide-producing bacteria-free cells | 2.3 log CFU/cm2 2.7 log CFU/cm2 | Gong and Jiang (2015) |
| Stainless steel Plastic | Hydrogen sulphide-producing bacteria biofilms | 2 log CFU/cm2 1.5 log CFU/cm2 (6 h/30°C) | ||
| LISTEXP100 | Stainless steel wafers | Listeria monocytogenes biofilm | Complete elimination (24 h/20°C) | Iacumin, Manzano and Comi (2016) |
| (108 PFU/ml) | ||||
| Pre-harvest | ||||
| Phage | Animal/crop | Target microorganism | Reduction | Reference |
| UAB_Phi20, UAB_Phi78, UAB_Phi87 encapsulated in liposomes (1011 PFU/ml) | Broiler chickens | Salmonella | ∼4 log CFU/g (6 d post-infection) | Colom et al. (2015) |
| CEV1, CEV2(1011 PFU/ml) | Sheep | Resident Escherichia coli O157:H7 | 99.9% | Raya et al. (2011) |
| CP14 (Group III) | Broiler chickens | Campylobacter jejuni | >3 log CFU/ml | Hammer et al. (2014) |
| CP68 (Group II) | ||||
| Myoviridae | Potato | Dickeya dadantii | No disease progression | Soleimani-Delfan et al. (2015) |
| Siphoviridae | ||||
| Post-harvest . | ||||
|---|---|---|---|---|
| Direct application of phage . | ||||
| Bacteriophage . | Food type . | Target microorganism . | Reduction (storage time/temp) . | Reference . |
| ListShield (106–108 PFU/ml) | Lettuce | Listeria monocytogenes | 91% (5 min) | Perera et al. (2015) |
| Cheese | 82% (5 min) | |||
| Smoked Salmon | 90% (24 h/4°C) | |||
| Frozen entrèes | 99% (24 h) | |||
| Apple slices | 93% (24 h/4°C) | |||
| Phage OSY-SP | Cut green pepper | Escherichia coli O157:H7 | 2.4–3 log CFU/g (3 d/4°C or 4 h/25°C+4°C/3 d) | Snyder, Perry and Yousef (2016) |
| Spinach leaves | 3.4–3.5 log CFU/g (3 d/4°C or 4 h/25°C+4°C/3 d) | |||
| Group II virulent phage (108 PFU/ml) | Chicken liver (homogenised) | Campylobacter jejuni | 0.2–0.7 log CFU/g (48 h/4°C) | Firlieyanti, Connerton and Connerton (2016) |
| SalmoFresh (107 PFU/ml) | Turkey breast cutlets | Salmonella enterica serotype Heidleberg | 1.3 log CFU/g (7 d/4°C) | Sharma, Dhakal and Nannapaneni (2015) |
| Phage FWLLm1 (MOI = 100) | Milk | Listeria monocytogenes | Below detection limit (10 d/4°C) | Rodríguez-Rubio et al. (2015) |
| + Coagulin C23 (584 AU/ml) | ||||
| P100 (2.3 × 107 PFU/ml) | Salmon | Listeria monocytogenes | Below detection limit (1 d/4°C) | Baños et al. (2016) |
| + Enterocin AS-48 (0.37 μg) | Hake | Below detection limit (2 d/4°C) | ||
| Phage-containing antimicrobial packaging | ||||
| Bacteriophage + type of Packaging | Food type | Target microorganism | Reduction (storage time/temp) | Reference |
| Escherichia coli phage + modified cellulose membranes | Cooked turkey breast | Escherichia coli O157:H7 | (All 15 d/4°C) | Anany et al. (2011) |
| Aerobic storage | ∼1.2 log CFU/g | |||
| Modified atmospheric | ∼2 log CFU/g | |||
| Packaging | ||||
| Vacuum | >4 log CFU/g | |||
| Escherichia coli phage + paper coated with encapsulated phage or impregnated with phage suspension | Alfalfa seeds Alfalfa sprouts | Escherichia coli O104:H4 | Below detection limit(1 h)1 log (5 d/RT) | Lone et al. (2016) |
| LISTEXP100 + immobilised on modified cellulose membranes | Cooked turkey | Listeria monocytogenes | >1 log CFU/cm2(25 d/4°C) | Lone et al. (2016) |
| P22 (108 PFU/ml) | Stainless steel | Salmonella Typhimurium biofilm formation | >90% (24 h) | Karaca, Akcelik and Akcelik (2015) |
| ∼90% (48 h) | ||||
| ∼85% (72 h) | ||||
| Salmonella Typhimurium preformed biofilm: 72 h48 h24 h | <10%∼35%>65% | |||
| Phage cocktail (108 PFU/ml) | Stainless steel Plastic | Hydrogen sulphide-producing bacteria-free cells | 2.3 log CFU/cm2 2.7 log CFU/cm2 | Gong and Jiang (2015) |
| Stainless steel Plastic | Hydrogen sulphide-producing bacteria biofilms | 2 log CFU/cm2 1.5 log CFU/cm2 (6 h/30°C) | ||
| LISTEXP100 | Stainless steel wafers | Listeria monocytogenes biofilm | Complete elimination (24 h/20°C) | Iacumin, Manzano and Comi (2016) |
| (108 PFU/ml) | ||||
| Pre-harvest | ||||
| Phage | Animal/crop | Target microorganism | Reduction | Reference |
| UAB_Phi20, UAB_Phi78, UAB_Phi87 encapsulated in liposomes (1011 PFU/ml) | Broiler chickens | Salmonella | ∼4 log CFU/g (6 d post-infection) | Colom et al. (2015) |
| CEV1, CEV2(1011 PFU/ml) | Sheep | Resident Escherichia coli O157:H7 | 99.9% | Raya et al. (2011) |
| CP14 (Group III) | Broiler chickens | Campylobacter jejuni | >3 log CFU/ml | Hammer et al. (2014) |
| CP68 (Group II) | ||||
| Myoviridae | Potato | Dickeya dadantii | No disease progression | Soleimani-Delfan et al. (2015) |
| Siphoviridae | ||||
Phage are highly specific, and more often than not are strain specific. As a consequence, their use in food safety and quality applications should have minimum, if any, impact on the wider microbiota following consumption. Most phage will be inactivated during gastric transit, considering that most are inactivated at pH values below 4. However, the ability of bacteria and phage to antagonistically co-evolve is a concern for the use of phage in food biocontrol. Bacteria harbour a diverse range of phage resistance mechanisms to which phage can evolve against and escape (Samson et al.2013) resulting in escalation of defence and counterdefence cycles. This co-evolution has been termed the arms race dynamic (ARD) and is generally observed early in phage–host interactions. In terms of ensuring food safety, the use of phage cocktails can increase their spectrum of inhibition and reduce the risk of phage-resistant mutants. Moreover, the acquisition of phage resistance in a bacterial cell can be associated with a cost in terms of growth rate or virulence. As a specific example, Meaden, Paszkiewicz and Koskella (2015) revealed that phage resistance in the plant pathogen Pseudomonas syringae resulted in a substantial cost in terms of growth when in its tomato plant host with reduced densities recorded relative to the phage sensitive strain but which was not observed in nutrient-rich media.
Furthermore, it is thought that phage have a stronger evolutionary potential owing, in part, to their shorter generation times and larger population sizes (Gandon and Michalakis 2002). Based on this concept, it has been possible to produce improved phage in terms of host range and infectivity through a process of phage ‘training’ which simply involves serial passage of the phage in the same clonal host population whereby the phage can rapidly evolve to overcome the initial steps of phage resistance (Ebert 1998). Betts et al. (2013) describe this method as ‘evolving phages into the future’ before confronting the improved phage with its bacterial past. However, the success of the process can be limited by the occurrence of an ARD. Despite this, phage training has produced successful results for certain phage–host pairs and certainly warrants consideration when producing phage for food biocontrol purposes. For example, five cycles of serial passage (in liquid culture) of PAK-P3 phage on a clinical isolate of Pseudomonas aeruginosa improved its in vivo efficacy and its host range on a panel of 20 P. aeruginosa cystic fibrosis strains (Morello et al.2011). In contrast, Scanlan et al. (2013) reported that while co-evolved variants of the Pseudomonas fluorescens phage SBW25Φ2 were capable of infecting sympatric and allopatric co-evolved variants of its host strain P. fluorescens SB25, they were unable to infect alternative P. fluorescens strains suggesting genetic constraints may exist for certain phage. Sandia National Laboratories (Albuquerque, New Mexico) have developed a phage host-range expansion technology which claims to increase the host range of phage, reduce the number of phage required for cocktails and can mutate known phage strains for specific bacteria of interest. This technology is available to license for commercial use for food safety, medical and veterinary applications (Sandia National Laboratories 2016; https://ip.sandia.gov/technology.do/techID=167).
With a history of over a century of safe use in phage therapy, there is a body of evidence to suggest that phage are not harmful to eukaryotic hosts. However, Hagens and Loessner (2010) compiled a list of properties which they suggest phage should have in order to be applied in food which includes the following; should have a broad host range, in that they kill members of the target species and/or genus; should be strictly lytic; should be incapable of transduction; should possess no undesirable genes (e.g. allergenic proteins, pathogen-associated proteins); should have a known genome sequence; should be propagated on a non-pathogenic host; should be stable over storage and application; should be amenable to commercial production; should have GRAS status; and proof should be provided of no adverse effects through oral feeding studies. While the majority of these properties are essential prerequisites for phage destined for food safety purposes, it is most probable that phage cocktails will be required to ensure a broad spectrum of inhibition. In addition, the availability of non-pathogenic hosts may be a limitation in using certain phage for food safety applications, again considering the narrow spectrum of most phage. Attenuated derivatives of pathogenic strains may provide useful surrogates in this instance.
Unlike other antimicrobial agents, phage numbers increase during the lytic lifecycle generating progeny phage which also participate in target killing. Lysis can occur minutes or hours following phage adsorption (Guttman, Raya and Kutter 2005) and is dependent on phage type, environmental conditions and physiological activity of the host. Ideally, phage require actively replicating bacterial cells for the lytic lifecycle. However, ‘lysis from without’ (LO) can occur when a large number of phage (more than 100) attach to the bacterial cell and in the absence of phage replication cause membrane swelling and bulging, subsequent hole formation, cytoplasmic leakage and cell death (Tarahovsky, Ivanitsky and Khusainov 1994; Abedon 2011; Kazi and Annapure 2016).
Post-harvest phage applications
Direct application of phage to food
Direct application methods include spraying food with phage, dipping food in a phage preparation or adding phage as liquid to a large volume of food (Hagens and Loessner 2010). There are important considerations when using phage for post-harvest food applications: first most foods are solid and hence the surface microstructure will influence phage diffusion rates, bacteria may not be readily accessible to phage and bacterial numbers will most likely be low assuming good hygienic standards have been met (Hagens and Loessner 2010). In addressing these issues, Hagens and Loessner (2010) suggest that when target cells are a limiting factor, it is necessary to add a sufficiently high number of phage to ensure rapid contact between the two and propose a threshold of ∼1 × 108 plaque forming units (PFU)/ml. Moreover, the authors suggest that the optimal point of application is when bacterial contamination is recent and the application is most convenient, economical and least invasive to the food process. As with bacteriocins, phage should be tested in their target food environment under conditions mimicking realistic scenarios of contamination and processing (Hagens and Loessner 2010).
A collection of four phage was assessed individually and as a cocktail for their ability to control E. coli O157:H7 on beef samples at 4°C, 22°C and 37°C (Liu et al.2015). Pathogen reduction strongly correlated with multiplicity of infection (MOI: number of phage per cell) where a higher MOI (1000) was necessary for inhibition to occur. Interestingly, phage T5 was the most effective at all tested temperatures and proved even more effective than a cocktail containing T5 for controlling the pathogen on beef. The authors conclude that the efficacy of the cocktail was reduced as a result of competitive interference between the phage whereby one phage prevents replication of another. In this regard, it is important to ensure that phage within a cocktail are compatible with each other and should, at the least, exhibit the same killing activity as each individual phage. Synergistic activity between phage in a cocktail has also been observed whereby one phage facilitates the infection process of another (Weber-Dąbrowska et al.2016). The study by Liu et al (2015) also revealed that the phage are capable of replication at refrigeration temperature although with increased latent periods suggesting their suitability for biocontrol of E. coli O157:H7 on beef during storage or transport.
The nature of the food environment can be a significant factor in phage biocontrol strategies whereby phage incorporation into the food matrix may require higher phage titres than when applying the phage to the surface owing to the reduced probability of phage-target bacterium contact. This was observed by Sharma, Dhakal and Nannapaneni (2015) when they applied SalmoFresh (Intralytix Inc), an anti-Salmonella enterica phage preparation, to the surface of raw turkey breast and to ground turkey breast. Indeed, at 4°C the phage preparation (107 PFU/g) significantly reduced Salmonella numbers by 0.8, 0.6 and 1.6 log10 CFU/g on days 0, 1 and 7 of storage, respectively, on raw turkey breast when compared to the control, but failed to reduce Salmonella numbers in contaminated ground turkey which had been treated with phage before grinding. The authors concluded that the increased surface area in the ground meat required greater phage numbers to reach target cells.
Snyder, Perry and Yousef (2016) investigated whether the incubation temperature of phage-treated foods has an impact on phage efficacy, considering that the phage lytic cycle requires metabolically active cells. In this case, E. coli O157:H7 phage, OSY-SP, isolated from municipal waste water, proved equally effective for reducing pathogen numbers on cut pepper and spinach leaves following storage of phage-treated samples for 72 h at 4°C or a combination of 25°C for 4 h prior to refrigerated storage (to aid host outgrowth) (Snyder, Perry and Yousef 2016). The authors speculated that pathogen reduction at the refrigerated temperature may be due in part to LO or to the initial production of progeny phage even if bacterial growth is slow.
Combining phage with other antimicrobial treatments has generated impressive results. For example, the combination of the Listeria phage FWLLm1 (MOI of 100) with the bacteriocin coagulin C23 (584 AU/ml) was found to be more effective than either treatment alone for reducing L. monocytogenes numbers in milk (Rodríguez-Rubio et al. 2015). Indeed, an initial inoculum of 5 × 104 CFU/ml of the pathogen was reduced to below detection limits (less than 10 CFU/ml) by day 4 of milk storage at 4°C. While resistant mutants were recovered from the combination treatment at the end of the 10 day storage period, the fraction of resistant mutants was lower than from either individual treatment. As a surface treatment, SalmoFresh (Intralytix Inc) (109 PFU/ml) reduced Salmonella numbers on chicken breast fillets by 0.8, 0.8 and 0.1 log CFU/g following 0, 1 and 7 days of storage, respectively, at 4°C under aerobic conditions and was found to be even more effective when used in combination with modified atmospheric packaging (95% CO2/5% O2) resulting in 1.2, 1.1 and 1.2 log reductions in Salmonella numbers on days 0, 1 and 7, respectively (Sukumaran et al. 2016). The combination of ListexP100 (Microes BV) with the chemical antimicrobials, potassium lactate and sodium lactate on cooked turkey and roast beef revealed that the phage preparation provides an additional hurdle for RTE beef and turkey contaminated with L. monocytogenes (Chibeu, Agius and Gao 2013). This was particularly apparent for cooked turkey at 4°C but not at 10°C. Indeed, Listeria numbers were reduced by ∼4 log CFU/cm2 on cooked turkey in the presence of ListexP100 (107 PFU/cm2) and potassium lactate (2.8%) when compared to the control following 28 days of storage at 4°C. Either treatment alone resulted in ≤1.0 log reductions over the same storage period revealing an additive effect. On roast beef, phage provided an additional hurdle to the combination of potassium lactate (2.8%) and sodium lactate (0.2%) at 10°C following 28 days of storage which was not apparent at 4°C.
Phage-containing antimicrobial packaging
Phage-containing antimicrobial packaging is a particularly appealing option for phage biocontrol strategies as it provides a protective and contained environment for the phage along with their sustained release onto the food surface. Spraying applications, on the other hand, unavoidably emit phage particles into the surrounding environment resulting in unnecessary phage waste and dipping runs the risk of cross contamination between batches of food (Lone et al.2016). Anany et al. (2011) immobilised phage onto the surface of modified cellulose membranes based on the charge differences between the phage head and tail, whereby the net negative charge of the head aided phage attachment to the positive surface of the charged membrane, leaving the tail (which emits an overall positive charge) free to attach to target cells. The phage-containing cellulose membranes proved effective for controlling the growth of E. coli O157:H7 and L. monocytogenes growth on raw meat and RTE meat under various storage temperatures and packaging conditions.
Prototype bioactive packaging materials were developed by (i) immobilising phage onto positively charged modified cellulose membranes, (ii) impregnating paper with a phage suspension and (iii) encapsulating phage in alginate beads followed by application of the beads onto paper (Lone et al.2016). The various bioactive materials were compared with free phage for L. monocytogenes control on cantaloupes and RTE meats and E. coli O104:H4 control on alfalfa seeds and sprouts. Free phage proved more effective for pathogen control on cantaloupes with L. monocytogenes numbers falling below the detection limit after 5 days of storage at 4°C and 12°C, while at 25°C L. monocytogenes numbers were below the detection limit after 3 and 6 h of application, highlighting the greater efficacy of phage when cells are metabolically active. In contrast, immobilised phage resulted in only a 1 log reduction in L. monocytogenes numbers following 5 days of storage at all temperatures. The authors suggest that the success of the free phage suspension was possibly due to its direct application to the contaminated spot on the cantaloupe sample, whereas the immobilised phage covered the whole sample thus reducing phage numbers available to the localised bacterial cells. Moreover, limited diffusion of the phage within the cantaloupe may also have been an issue. However, the immobilised phage proved as effective as free phage for controlling L. monocytogenes growth on RTE cooked turkey at 4oC and 10°C over the 25-day storage period. Likewise, the immobilised and free phage exhibited similar antimicrobial activity against E. coli on alfalfa seeds and sprouts, bringing about a reduction below the detection limit after 1 h of phage exposure in seeds and a 1 log reduction in sprouts compared to the control after 5 days at room temperature. Importantly, all three prototype materials exhibited potential as bioactive packaging for food safety applications.
More recently, absorbent phage-containing food pads were developed for intended use in chilled meat trays to extend shelf life (Gouvêa et al. 2016). In this case, a mix of six Salmonella typhimurium phage was added to the pads by dripping onto the surface. The efficacy of the pads was tested following contact with S. typhimurium-containing agar at incubation temperatures of 10°C and 15°C. At both temperatures, phage diffusion into the agar occurred. The inhibitory effect was most obvious at 15°C when the bacterial cells were more metabolically active and distinct clearing in the agar was observed. Exposure of the pads to Salmonella inoculated broth revealed that Salmonella reduction strongly correlated to phage numbers where the pad containing the highest phage number (109 PFU/ml) resulted in the largest log reduction (4.36 log cycles) following 12 h of incubation at 15°C. Moreover, the phage remained viable within the pads for the 48-h experimental period.
Biosanitation
Commercial phage preparations for controlling pathogen contamination on foods can also be used as surface sanitisers. ListShield (Intralytix Inc) is marketed as an ‘all-natural non-chemical antimicrobial preparation’ for eliminating or significantly reducing L. monocytogenes contamination on various foods (RTE meats, fruit, vegetables, dairy, etc.) as well as food contact surfaces. ListexP100 (Micreos BV) is also recommended for L. monocytogenes elimination or reduction on foods such as cheese, meat and fish and food contact surfaces. Indeed, ListexP100 at a concentration of 8 log PFU/cm2 was recently shown to completely eliminate 2 log CFU L. monocytogenes/cm2 on dry-cured ham slices at 4°C, 10°C and 20°C and eliminated L. monocytogenes cells and biofilms from machinery surfaces used for the dry-cured ham preparation (Iacumin, Manzano and Comi 2016). The ability of S. typhimurium to form biofilms on polystyrene and stainless steel surfaces was significantly reduced in the presence of phage P22 at 106 PFU/ml (Karaca, Akcelik and Akcelik 2015). However, the phage proved less effective at eliminating pre-existing biofilms. Moreover, Viazis, Labuza and Diez-Gonzalez (2015) estimated that it would take the phage cocktail BEC8 ∼2 to 4 h to generate a 5-log reduction of liquid E. coli O157:H7 cells depending on the surface if initial cell numbers were high. In a recent review of the literature, Gutiérrez et al. (2016) assessed the potential of phage and phage-derived proteins for biofilm eradication and concluded that phage disinfectants have a lot to offer in terms of food safety but more research is required for scale-up and manufacturing and a regulatory framework should be established. Biofilms can be impenetrable to phage; however, some phage harbour polysaccharide depolymerases with the capacity to hydrolyse polysaccharides or polysaccharide derivatives (Pires et al.2016) and which promote phage invasion and dispersion throughout the biofilm. These enzymes have been associated with tail-spike proteins (Barbirz et al. 2009). Some phage harbour virion-associated peptidoglycan hydrolases which can be located at the baseplate of the phage and cause the LO phenomenon when high numbers of phage are present (Moak and Molineux 2004), hence aiding biofilm invasion. Phage-encoded endolysins also offer potential as antimicrobials against biofilms as they target peptidoglycan when added externally to Gram-positive cells (Fischetti 2008). Combining the endolysin with a polycationic peptide capable of penetrating the outer membrane of Gram negatives has also resulted in the development of highly effective antimicrobials termed ‘Artilysins’ (Briers et al. 2014a,b). However, application of the latter systems in the near future is unlikely given the current climate of consumer opposition to genetic manipulation.
Pre-harvest phage applications
It is generally accepted that pre-harvest phage applications may not completely eliminate the target pathogen (Hagens and Loessner 2010). However, it is still a worthwhile approach for decreasing pathogen levels given that it has been estimated that a one log10 reduction in pathogen load at the pre-slaughter stage has the potential to reduce consumer risk of food poisoning by 45% and a two log10 reduction could reduce the risk by 75% (Havelaar et al.2007). Furthermore, it is likely that the target bacterium will be in a state of active replication, thus allowing the use of lower phage doses (Hagens and Loessner 2010). Owing to the exponential rate of pathogen spread amongst animals and the plethora of sources of pathogen contamination, it is advised to use broad-spectrum phage and phage cocktails which target multiple strains and to use these in rotation to reduce the risk of phage-resistant mutants (Hagens and Loessner 2010).
As cattle hides are considered the main source of E. coli O157:H7 contamination on beef carcasses during processing, Arthur et al. (2017) investigated the efficacy of applying a commercial E. coli O157:H7 phage preparation to cattle as they entered the lairage area of a commercial beef processing plant. In this study, 289 cattle received phage spray and 301 served as untreated controls and hide and carcass samples were later investigated for E. coli O157:H7 prevalence and concentration. Phage treatment of cattle hide did not result in significant reductions in E. coli O157:H7 in comparison to the untreated animals where cattle hides treated with phage spray had a pathogen prevalence of 51.8% compared to 57.6% in the untreated animals. E. coli O157:H7 prevalence on treated carcasses was recorded at 17.1% compared to 17.6% in the untreated control. Oral delivery of a phage cocktail to cattle orally inoculated with E. coli O157:H7 failed to reduce faecal shedding of the pathogen in comparison to the untreated animals (Rivas et al.2010). Moreover, polymer encapsulated phage failed to reduce E. coli O157:H7 shedding in feedlot cattle although the duration of shedding was reduced by 14 days in bolus-fed steers (Stanford et al.2010). In contrast, a two-phage cocktail composed of CEV1 and CEV2 phage proved effective at reducing resident E. coli O157: H7 from sheep, resulting in a >99.9% reduction (Raya et al.2011). However, sheep found to naturally harbour CEV2 had the lowest levels of E. coli O157: H7 with an ∼99.99% reduction in pathogen numbers when compared to the untreated, phage-free control. While pre-harvest in vitro experiments for phage biocontrol of E. coli O157:H7 have generated promising results, the varied results from in vivo studies have been recently reviewed by Sabouri et al. (2016) who propose that the adverse conditions of the gastrointestinal tract or the inability of phage to reach target bacteria may represent obstacles to phage efficacy and must be overcome through novel strategies.
The results have been more promising with regard to phage use for pathogen biocontrol in poultry with treatment schedules having an impact on phage efficacy. A cocktail of three broad-spectrum phage against S. enterica serovar Typhimurium and S. enterica serovar Enteritidis proved most effective for reducing S. typhimurium load in pre-inoculated chickens when orally administered either 1 day prior to pathogen infection or on the day of infection and for a number of days afterwards (up to 15) resulting in up to 4.4 log10 reductions in the chicken cecum (Bardina et al.2012). In contrast, phage treatment on days 4 and 5 post-infection achieved a 1 log10 reduction when compared to the control.
Intercloacal inoculation of 1012 PFU/ml of phage st1 in chickens 1 h after challenge with 1010 CFU/ml of S. enterica serovar Typhimurium resulted in a 2.9 log reduction in pathogen numbers in cloacal swabs 6 h after treatment and the pathogen could not be detected after 24 h (Wong et al.2014).
Hammer et al. (2014) found that using phage with different infection kinetics, for example, the group II and group III phage against Campylobacter jejuni, proved more effective for pathogen elimination than using one phage type. In this regard, oral administration of the group III phage to C. jejuni contaminated broiler chickens followed by administration of the group II phage 24 h later resulted in more than 3 log reductions in pathogen numbers in faeces. In contrast, the simultaneous application of two group III phage failed to elicit a reduction in pathogen numbers presumably due to the emergence of resistant mutants.
Phage encapsulation should ensure phage survival in the adverse environment of the gastrointestinal tract and should increase the efficacy of pre-harvest phage biocontrol strategies. In this regard, a liposome encapsulated Salmonella phage cocktail proved significantly more stable in simulated gastric juice than non-encapsulated phage and improved phage retention in the chicken gastrointestinal tract, with encapsulated phage being detected in the cecum samples of 38.1% of chickens 72 h after oral administration compared to only 9.5% of chickens harbouring non-encapsulated phage (Colom et al.2015). Interestingly, non-encapsulated phage and encapsulated phage performed equally well in reducing Salmonella numbers in the experimentally infected chickens following daily administration for 6 days. However, while the protective effect of the non-encapsulated phage disappeared once treatment stopped, the inhibitory effect of the encapsulated phage persisted for at least 1 week after treatment.
Phage have also shown promise for controlling plant diseases (Jones et al.2012). They have received much interest in this regard as a result of their environmentally friendly nature and because traditional chemicals and antibiotics are losing efficacy due to bacterial resistance development (Buttimer et al.2017). However, using phage for plant pathogen control can be fraught with obstacles as a consequence of environmental conditions. Phage are naturally sensitive to UV, which is undoubtedly one of the greatest hurdles to their stability on plant surfaces (Jones et al. 2012).
As an example, Yu et al. (2016) recently characterised phage active against P. syringae pv. actinidiae which causes bacterial canker disease in kiwi fruit. The five phage were found to be stable at 50°C, at pH 11 and under UV-B light suggesting that they should retain stability in the natural growth environment of the kiwifruit tree; this study thus highlights the range of properties required for phage intended for plant pathogen control purposes.
Four virulent phage active against Xylella fastidiosa subsp. fastidiosa (Xf), the causative agent of Pierce's disease in grapevines, proved capable of significantly reducing pathogen load in the plant whether applied therapeutically or prophylactically and symptoms of the disease regressed 1 week after therapeutic treatment (Das et al.2015). As phage-resistant mutants were not found in planta, the authors generated phage-resistant mutants in vitro of which a number were shown to exhibit adsorption defects for all four phage. Interestingly, these mutants failed to elicit disease symptoms in grapevines; thus, the authors concluded that phage receptors in X. fastidiosa may be essential for virulence.
The commercially available phage product AgriPhage (Omnilytics, Utah, USA) is active against Xanthomonas campestris pv. vesicatoria and P. syringae pv. tomato, the causative agents of bacterial spot on pepper and tomato plants. Omnilytics recommends that the product is applied once weekly during the growing period, where early morning, late afternoon and nighttime applications provide best results. Indeed, scheduled applications avoiding bright sunlight can reduce the adverse effects of UV (Jones et al.2012). Using non-pathogenic-sensitive strains and protective formulations have also been shown to increase phage persistence and stability on plant surfaces (Jones et al.2012). For example, the application of an attenuated mutant of Xanthomonas perforans on tomato foliage prior to phage application significantly improved phage persistence on foliar tissues in both greenhouse and field experiments (Iriarte et al.2012). Formulated X. campestris phage in either pre-gelatinised corn flour (PCF) + 0.5% sucrose or 0.5% Casecrete NH-400 + 0.5% sucrose + 0.25% PCF proved more effective than non-formulated phage for reducing bacterial spot disease in three consecutive field trials (Balogh et al.2003).
THE GUT MICROBIOTA AND THE NEED FOR NARROW-SPECTRUM ANTIMICROBIALS
The human gut microbiota is composed of a diverse community of bacteria, archaea, viruses, yeasts and fungi (Mai and Draganov 2009). Bacterial numbers are estimated at 1013–1014 cells (Ley, Peterson and Gordon 2006; Guinane and Cotter 2013). Reports on the number of microbial species in any given individual vary from ∼100, based on culture-dependent techniques (Faith et al.2013), to ∼160, based on culture-independent techniques. In general, the majority belong to two phyla, the Firmicutes (35%–80%) and the Bacteroidetes (17%–60%) (Qin et al.2010). The gut microbiota provides several vital functions to its host including vitamin synthesis, metabolism of drugs and xenobiotic compounds, modulation of the immune system, inhibition of pathogens and maintaining the structure and function of the gastrointestinal tract (Jandhyala et al.2015).
It is difficult, if not impossible, to define what constitutes a healthy microbiota owing to the remarkable variation observed between individuals (Human Microbiome Project Consortium 2012); however, it is generally accepted that an optimal microbiome will have a high level of species diversity (Heiman and Greenway 2016) and will be relatively stable over time (Faith et al.2013). More recently, Falony et al. (2016) identified a global human core microbiota consisting of 14 genera using data from almost 4000 individuals but concluded that total gut microbiota diversity is still underexplored. Changes in microbial composition and abundance have been associated with a range of human diseases, a topic which has been recently reviewed by Li et al. (2016). For example, decreased microbial diversity has been observed in the intestinal microbiota of those suffering from inflammatory bowel disease (IBD) (Hansen et al.2010). Patients with type 2 diabetes exhibited an increased abundance of the Betaproteobacteria class, a higher Bacteroidetes/Firmicutes ratio and lower levels of Clostridia when compared to healthy individuals (Larsen et al.2010). Another study involving 345 Chinese individuals revealed a decreased abundance of some universal butyrate-producing bacteria, an increase in opportunistic pathogens as well as an enrichment of certain microbial functions including oxidative stress resistance and functions involved in sulphate reduction in type 2 diabetes patients (Qin et al.2012). Other diseases which have been associated with altered gut microbiota and microbiome profiles include irritable bowel syndrome, liver diseases, cardiovascular disease, type 1 diabetes, autism, multiple sclerosis and obesity (Li et al.2016).
One factor which has been directly linked to alterations of the gut microbiota is antibiotic administration (Francino 2016) and ∼68 human prescribed antibiotics (alone, as combinations or cocktails) have been associated with such effects (Ferrer et al.2016). Broad-spectrum antibiotic treatment has been shown to dramatically reduce microbiota diversity (Dubourg et al.2014) and affect major changes at the level of gut microbiota metabolism (Perez-Cobas et al. 2013). Dethlefsen et al. (2008) found that about 30% of the bacterial community was influenced by antibiotic treatment resulting in a decrease in taxonomic richness, diversity and evenness. A later study revealed that the antibiotic ciprofloxacin had a profound and rapid effect on the gut microbiota within 3 to 4 days of drug initiation resulting in loss of diversity and a shift in community composition (Dethlefsen and Relman 2011). While microbial communities began to return to their original state 1 week after treatment, they generally remained altered from their initial state. Indeed, gut microbiota alterations as a result of antibiotic treatment can last for months or even years (De La Cochetiere et al. 2005; Jernberg et al.2007; Dethlefsen et al.2008; Dethlefsen and Relman 2011). The consequences of these alterations can be both acute and long term, exposing patients to the immediate threat of intestinal infections, such as C. difficile-associated diarrhoea, or indirectly impacting long-term health through disruptions to the gut microbiota which may be associated with a range of diseases (Francino 2016; Li et al.2016).
Moreover, the human gut resistome, a term which describes the antibiotic resistance genes of the gut microbiota, is a significant concern with several studies reporting on the alarmingly high frequencies of such genes harboured within the human microbiota (van Schaik 2015; Francino 2016). Using metagenomic data from 252 faecal metagenomes from three different countries, Forslund et al. (2013) revealed that the most abundant resistance determinants were for antibiotics also used in animals and for those which have been available longer, and showed that these antibiotic resistance determinants can persist in an individual for at least a year. In a more recent study involving 1267 subjects from four countries on three continents, the relative abundance of antibiotic resistance genes was found to be less in those countries which have implemented restrictive legislation on antibiotic usage than in countries which have delayed such policies (Yang et al.2016). Given the wide-ranging complications associated with antibiotic use, it is hardly surprising that narrow-spectrum antimicrobials such as phage and bacteriocins can be regarded as highly appealing substitutes.
BACTERIOCINS IN GUT HEATH
With their narrow spectrums of inhibition and potent antimicrobial activities, bacteriocins have the capacity to endow the gut microbiota with a range of benefits from pathogen inhibition to potentially re-shaping microbiota composition. A specific gut pathogen which has been the focus of much bacteriocin-based research is C. difficile. This spore-forming, toxin-producing, opportunistic pathogen is considered a member of the normal gut microbiota in a minority of individuals but its growth is restrained by other dominant members (Bien, Palagani and Bozko 2013). Indeed, it has been reported to exist in up to 3% of healthy adults and 90% of healthy newborns (NCEC 2014). However, disruption of the normal microbiota, as occurs during antibiotic therapy, provides C. difficile with the opportunity to proliferate—presumably as a result of diminished competition (De La Cochetière et al.2008; Culligan and Sleator 2016). This can result in symptoms ranging from mild diarrhoea to potentially fatal colitis (NCEC 2014). In the USA alone, it is reported to be responsible for over 400 000 infections and ∼29 000 deaths on an annual basis and is the most common cause of nosocomial diarrhoea in the developed world (Culligan and Sleator 2016). Thus, it could be argued that C. difficile is part of the normal gut microbiota in the majority of individuals given the high incidence of C. difficile infection. Treatment generally involves broad-spectrum antibiotic therapy, such as vancomycin or metronidazole, which can cause other complications such as the enrichment of resistant isolates and the spread of antibiotic resistance genes (Sood et al.2008). The development of alternative treatments to combat this highly problematic pathogen is urgently required.
In a screen of over 30 000 gut isolates, Rea et al. (2010) identified a two-component bacteriocin with potent activity in the nanomolar range against C. difficile. The bacteriocin, known as thuricin CD, is a member of the sactibiotic subclass of bacteriocins and is produced by Bacillus thuringiensis. While the bacteriocin is capable of killing a wide range of C. difficile isolates, it has minimum impact on gastrointestinal commensals. In a distal colon model, thuricin CD was found to be as effective as vancomycin and metronidazole at killing C. difficile but unlike the antibiotics which caused a decrease in the abundance of Firmicutes and Bacterioidetes and an increase in Proteobacteria, the bacteriocin had no significant impact on microbiota composition (Rea et al.2011). Bioavailability studies revealed that one peptide of the two-peptide bacteriocin is degraded by gastric enzymes pepsin and α-chymotrypsin; however, rectal administration of the bacteriocin to mice resulted in a >95% reduction of C. difficile shedding 1 h post-treatment when compared to the control, and a further 1.5 log reduction was observed 6 h after treatment (Rea et al.2014).
At a concentration of 260 μg/ml, nisin A was found to inhibit C. difficile in a model human colon but the bacteriocin also elicited temporary changes to the microbiota composition where Gram-positive bacteria were particularly affected (Le Lay et al.2015). Indeed, the most affected Gram-positive group, the Ruminococcaceae, decreased by 3.7 logs while an increase was observed in the Gram-negative Bacteroidetes and Enterobacteriaceae. However, the initial balance was restored 24 h after addition of the bacteriocin. In a later study, the minimum inhibitory concentration (MIC) of nisin A against several clinically isolated C. difficile strains was estimated at 6.2 μg/ml while that of nisin Z was estimated to be 0.8 μg/ml (Le Lay et al.2016). At a concentration of 25.6 μg/ml, nisin A was also found to reduce C. difficile spore viability by 40%–50% in a liquid suspension following 1 h of incubation at 37°C. Interestingly, a nisin Z-producing strain, which was shown to survive and proliferate in a simulated human colon, was incapable of inhibiting C. difficile (Le Lay et al.2015) suggesting that the producing strain may not be able to generate a sufficient concentration of bacteriocin to elicit an inhibitory effect.
The novel type B lantibiotic, NVB302, was found to be as effective as vancomycin at reducing C. difficile numbers in an in vitro gut model although C. difficile spores were unaffected by either treatment (Crowther et al.2013). Unlike the antibiotic, the bacteriocin had less detrimental impact on the Bacteroides fragilis group. The type II lantibiotic, lacticin 3147, was also found to be as effective as vancomycin and metronidazole at reducing C. difficile load in a human distal colon model (Rea et al.2011). However, the bacteriocin resulted in collateral damage similar to that of broad-spectrum antibiotics.
More recently, an R-type bacteriocin resembling the R-type ‘pyocins’ of P. aeruginosa was found to be produced by C. difficile and was called diffocin (Gebhart et al.2015). Diffocins contain contractile myophage-like sheath structures attached to receptor-binding proteins (RBPs) via tail fibres and a base plate (Nakayama et al.2000; Michel-Briand and Baysse 2002; Gebhart et al.2012). With its phage-like structure, diffocin was modified to inhibit the common C. difficile ribotype 027 strain (RT027) by replacing its natural RBP with a prophage-encoded RBP specific to RT027 that had been located through genome mining (Gebhart et al.2015). The modified bacteriocin was administered to mice via drinking water. It survived gastrointestinal passage and had no detectable impact on the gut microbiota and prevented antibiotic induced colonisation of C. difficile RT027-type spores (Gebhart et al.2015).
Another member of the intestinal microbiota which is capable of causing disease as a consequence of antibiotic therapy is Enterococcus faecalis. Indeed, enterococci harbour a vast array of resistance mechanisms to commonly used drugs (Shepard and Gilmore 2002) and pose a significant threat for immunocompromised individuals. Vancomycin resistant enterococci (VRE) can proliferate in the gut and displace normal microbiota, an event which precedes VRE invasion of the bloodstream (Ubeda et al.2010). Bacteriocin-21 is an enterococcal bacteriocin encoded on the conjugative plasmid pPD1 (Tomita et al.1997). An E. faecalis strain harbouring pPD1 was shown to replace the indigenous intestinal enterococci and outcompete non-pPDI carrying E. faecalis strains in a mouse model (Kommineni et al.2015). As the plasmid was capable of transfer to other E. faecalis strains, thus enhancing their survival, an E. faecalis strain carrying a conjugation-defective version of pPD1 was shown to clear the VRE without transfer of the plasmid, providing an example of how bacteriocin-producing commensals can influence niche competition in the gastrointestinal tract and in this case, eliminate drug-resistant bacteria without collateral damage (Kommineni et al.2015).
Microcin production by the probiotic bacterium E. coli Nissle 1917 (EcN) was recently shown to mediate competition among the Enterobacteriaceae in an inflamed gut (Sassone-Corsi et al.2016). Indeed, by limiting the growth of adherent-invasive E. coli, commensal E. coli and the pathogen S. enterica and colonising the inflamed gut, E. coli Nissle 1917 displaced the enteric pathogens from their niche. It is thought that EcN microcin is post-translationally modified at the C-terminus with a siderophore moiety and studies revealed that the EcN microcin genes are expressed in iron-limited medium but not in iron-rich medium (Sassone-Corsi et al.2016). The micocin therefore targets non-immune bacteria with particular siderophore receptors so does not significantly alter the microbiota.
Bacteriocins which create beneficial shifts in microbiota composition and abundance may provide a targeted approach for treatment of microbiota-associated diseases, particularly where a disrupted microbiota is a causative agent rather than a consequence of the disease. In a recent study, several class II bacteriocins were found to favourably modify the gut microbiota of mice (Umu et al.2016). The bacteriocins, which differed in terms of their inhibition spectrums and physico-chemical properties, were administered via their producing strains in drinking water and isogenic non-producing mutant strains served as controls. While the overall structure of the microbiota remained largely unaffected by the bacteriocin producers, certain transient but advantageous changes occurred at lower taxonomic levels. For example, the plantaricin bacteriocins inhibited Clostridium, the enterocins inhibited Staphylococcus and garvicin inhibited Enterococcaceae, and the sakacins, plantaricins and garvicin increased the proportion of LAB. In addition, garvicin treatment also coincided with decreased triglyceride levels in mice. The class IIb bacteriocin, bactofencin A, was also shown to subtly modulate microbiota in a simulated colon model creating beneficial alterations in terms of human health (Guinane et al.2016). At a concentration of 20 μM, the bacteriocin resulted in lower abundances of Clostridium spp. and Blautia and increased relative abundances of Streptococcus and Bifidobacterium, the latter which is known for its probiotic properties and is considered an important member of the healthy microbiota (Arboleya et al.2016).
In a specific example of how targeted manipulation of the microbiota may aid disease prevention or management, Murphy et al. (2013) investigated the efficacy of two gut microbiota-altering antimicrobials, vancomycin and the class II bacteriocin producer Lactobacillus salivarius UCC118, to impact metabolic abnormalities in diet-induced obese mice. Alterations in microbiota composition have been implicated in obesity-related metabolic dysregulation (Ley 2006; Li et al.2016). Indeed, it has been proposed that the gut microbiota impacts host metabolism through the extraction of energy and nutrients from food and by influencing the expression of host genes involved in metabolism (Li et al.2016). An increased abundance of Firmicutes and a decreased abundance of Bacteroidetes have been reported in the gut microbiota of genetically obese mice (Ley et al.2005; Turnbaugh et al.2008). However, the results in humans have been more conflicting with regard to the key microbial populations involved (Ley et al.2006; Duncan et al.2008; Schwiertz et al.2010; Le Chatelier et al.2013). As expected vancomycin and Lb. salivarius UCC118 altered the gut microbiota in the obese mice but with different outcomes. Vancomycin significantly reduced proportions of Firmicutes and Bacteroidetes and dramatically increased Proteobacteria and the vancomycin-fed mice gained less weight over the course of the study with lower fasting blood glucose, triglyceride levels and plasma TNFα compared with controls. In contrast, Lb. salivarius treatment failed to elicit an improvement in metabolic profiles and had no significant impact on Firmicutes though caused a relative increase in Bacteroidetes and Proteobacteria and a decrease in Actinobacteria. The authors suggest that while targeting the gut microbiota is a worthwhile therapeutic strategy, the specificity of the antimicrobial is critical to its success.
Interestingly, bioinformatic analysis has enabled the identification of multiple bacteriocin gene clusters in the human gut microbiome (Drissi et al.2015; Walsh et al.2015) where the most commonly identified were the >10 kDa class (bacteriolysins) followed by lantibiotics and sactipeptides (Walsh et al.2015). Drissi et al. (2015) reported 317 intestinal microbial genomes harbouring putative bacteriocins and proposed that those produced by the Firmicutes and the Bacteroidetes exhibited low antimicrobial activity and participated in permanent host defence against pathogen proliferation based on their physico-chemical properties. However, information regarding in vivo bacteriocin production in the gut is limited, yet strategies which induce bacteriocin production by the innate microbiota could prove highly effective for generating desirable shifts in microbiota composition or indeed eliminating pathogens through a form of ‘self-prophylaxis’. Guinane et al. (2015) revealed that the promoter of the class IId bacteriocin, bactofencin A which is produced by the porcine gut isolate Lb. salivarius, is induced in the presence of mild environmental stresses including low levels of salt and simulated gastric juice, while the promoters of the class IIb bacteriocins, also produced by Lb. salivarius gut isolates, are stimulated by bacteriocin-inducing peptide.
Combining oligosaccharides with probiotic bacteria isolated from probiotic yoghurt not only modulated bacterial growth but also enhanced bacteriocin production of certain strains (Pranckutè et al.2016). Likewise, the dairy starter L. lactis LMG 9450 was shown to induce bacteriocin production by probiotic strains during co-cultivation with the probiotics but was insensitive to the bacteriocin activity (Kos et al.2011).
BACTERIOPHAGE IN GUT HEALTH
We have reached an exciting juncture in phage research particularly in identifying the many possibilities for phage use in gastrointestinal health, from treating infection to modulating the microbiota and stimulating microbial diversity. Chiefly, their target-specific nature makes phage ideal candidates to protect the gut from gastrointestinal infections. Indeed, Felix d’Herelle, one of the key figures in phage discovery in the early 20th century, initially used phage to treat dysentery (Kutter 2008; Abedon et al.2011; Pirnay et al.2011). Successful phage treatment of Vibrio cholera and Salmonella infections have also been cited in the literature, although few, if any, involve blinded experiments (Abedon et al.2011). More recently, the Nestlé Research Centre in Switzerland and the International Center for Diarrhoeal Diseases Research in Bangladesh performed the first phase II, randomised, placebo-controlled, single-centre trial investigating the efficacy of phage treatment for childhood diarrhoea caused by E. coli (Sarker and Brüssow 2016). While the orally delivered phage survived gastric transit and could be recovered in the faeces, it did not perform better than the standard care for children hospitalised with acute diarrhoea (Sarker et al.2016). The phage failed to proliferate intestinally and the titres of E. coli in the faeces were found to be low, representing <5% of the total faecal microbial population, suggesting that higher oral phage doses may be required to ensure contact between phage and pathogen. Moreover, microbiota analysis of faecal samples of children enrolled in the trial revealed an abundance of faecal streptococci, which was shown to decrease while Bifidobacterium numbers increased in parallel with recovery from diarrhoea. This led the researchers to speculate if faecal streptococci were the causative agent of the diarrhoea. However, stool consistency as measured by the Bristol Stool Scale and colonic transit time have been shown to significantly impact faecal microbiota composition (Falony et al.2016; Tigchelaar et al.2016; Vandeputte et al.2016; Tottey et al.2017). Indeed, Vandeputte et al. (2016) observed that bacterial richness reached its minimum in diarrhoea-afflicted patients. It is therefore possible that the abundance of faecal streptococci in children suffering from diarrhoea may be a consequence of the diarrhoea itself given that streptococci are some of the dominant inhabitants of the small intestine (Zoetendal et al.2012) and the short transit time may have resulted in the enrichment of small intestinal microbiota while preventing colonic microbiota from reaching usual densities.
Using a mouse model, a phage cocktail (ShigActive, Intralytix Inc) was shown to reduce Shigella counts in mice which had been orally challenged with the susceptible strain (Mai et al.2015). The cocktail proved as effective as the antibiotic ampicillin at reducing Shigella colonisation and shedding but had significantly less impact on the gut microbiota. Studies of this nature highlight the potential success of phage therapy for gut infections once the aetiological agent is known and is sensitive to the intended phage treatment.
The phageome (total resident gut phage) is a relatively new field of microbiota research which has been greatly facilitated by the ongoing advances in high-throughput sequencing and data analysis technologies. The most abundant members of the gut phageome belong to the order Caudovirales and the tail-less single-stranded DNA lytic phage, the Microviridae (Reyes et al.2010; Kim et al. 2011), although the majority of phage sequences identified in the gut to date remain uncharacterised (Babickova and Gardlik 2015). It has been proposed that the ratio of phage to bacteria in the gut is 1:1 (Reyes et al.2010; Minot et al.2011; Ogilvie and Jones 2015), which reflects the temperate lifestyle of gut phage with the majority existing as prophage (Breitbart et al.2003; Reyes et al.2010; Minot et al.2011). Thus, phage are likely responsible for a significant degree of the genetic diversity observed in the gut microbiome. Indeed, the strain-specific DNA of pathogenic bacteria has been largely attributed to the presence of prophage sequences (Ohnishi, Kurokawa and Hayashi 2001; Banks, Beres and Musser 2002). Moreover, bacterial evolution is dramatically shaped by phage predation with genetic changes over time often associated with genes involved in preventing phage attachment (Reyes et al.2010). This suggests that phage may serve as tools to shape the microbiota and engender the diversity which is lost in certain disease states. Using a porcine model, Hong et al. (2016) investigated the impact of orally administered E. coli phage and an in-feed antibiotic on the host immune system and on surrounding microbial communities. In terms of the microbiota, both treatments resulted in significant abundance differences at the operational taxonomic unit (OTU) level, particularly for OTUs of the Lactobacillus and streptococcal genera. However, further studies are required to determine the consequence of these changes for the host. It remains to be seen whether phage in the form of multiphage cocktails will be able to modulate microbiota composition at phylum level.
Interestingly, phage concentrations in mucus layers have been shown to be greater than the surrounding environment with a phage to bacterium ratio of ∼20:1 recorded for the mucosal surface of the mouse intestine (Barr et al.2013). Barr et al. (2013) propose that the increased phage numbers protect mucosal surfaces from bacterial invasion whilst the phage have access to bacterial hosts, providing a non-host-derived immunity.
The adult phageome exhibits long-term stability with 80% of the observed phage sequences in an individual persisting for 2.5 years, although the lytic Microviridae phage were capable of rapid evolution to the point of becoming a new species (Minot et al.2013). While the virome (phage and viruses) was found to be unique to individuals, it was shown to respond dynamically to diet such that viromes of individuals on similar diets were seen to converge (Minot et al.2011). A healthy gut phageome consisting of the actively replicating phage was recently defined as a core collection of 23 phage found in over 50% of 64 healthy adults and 132 common phage found in 20%–50% of the adults (Manrique et al.2016). As this core phage collection was significantly reduced in sufferers of ulcerative colitis and Crohn's disease, the authors postulate that phage may be important for maintaining host health.
While there is a scarcity of studies examining gut phageomes in diseased states, the few which have been performed consistently reveal differences between healthy individuals and patients, a phenomenon which has been studied for IBD in particular and suggests that the phageome may represent a viable indicator of human health (Dalmasso, Hill and Ross 2014). Microscopy studies of biopsy samples obtained during colonoscopies revealed that sufferers of Crohn's disease had significantly more mucosal phage than healthy individuals (2.9 × 109 virus-like particles (VLPs) versus 1.2 × 108 VLPs, respectively) and ulcerated mucosa had less phage than non-ulcerated mucosa (2.9 × 109 VLPs v 4.1 × 109 VLPs, respectively) (Lepage et al.2008). Using high-throughput sequencing and metagenomic analysis, Wagner et al. (2013) identified a large abundance of phage in the ileum tissue and gut wash samples from paediatric patients of Crohn's disease and the most abundant viral sequences belonged to Caudovirales. Pérez-Brocal et al. (2013) observed lower diversity but more variability for the viromes and microbiomes of faecal samples obtained from Crohn's disease patients compared to healthy control samples. A significant expansion of Caudovirales phage was observed in intestinal preparations from Crohn's disease and ulcerative colitis patients which did not appear to be secondary to bacterial population changes (Norman et al.2015). Thus, the authors concluded that the virome contributes to or is a biomarker for IBD.
At this point more research is warranted before definitive conclusions can be made regarding the role of phage in IBD. However, given the role of phage in shaping microbial diversity in other communities (e.g. aquatic ecosystems; Sime-Ngando 2014) it is probable that phage play a significant role in shaping and maintaining microbial diversity in the gut. Insults which result in disruptions to the phage community and especially the endogenous prophage community (prophage induction) will undoubtedly trigger alterations to bacterial composition of which certain alterations will have unfavourable consequences for mammalian health.
PERSPECTIVE
The broad-spectrum nature of classic small molecule antimicrobials has been extraordinarily effective in helping clinicians to control infectious disease for many decades. Millions of lives have been saved as a result of these important therapeutics. But their use has not been without consequences, in terms of generating antibiotic resistance which threatens their very usefulness, and also in terms of collateral damage to microbiomes, including those of humans, animals and the environment. Research in recent years has confirmed that each of us exists as a finely tuned ecosystem with our associated microbial communities whose disruption can potentially have detrimental consequences for our health. Bacteriocins and phage are natural components of these microbial communities, aiding competition and survival, microbial diversity and fitness, and have a precision-killing nature that protects these delicate balances. It makes sense to harness these tools to control harmful and unwanted microbial growth in food and in the gut. But perhaps the greatest challenge to using narrow-spectrum antimicrobials is the very fact that they are so specific and the target bacterium should be known before treatment can be initiated. This is particularly true of phage where even the most broad-spectrum phage is usually more specific than the narrowest-spectrum antibiotic (Melo et al.2017). Strategically designed phage cocktails will aid in overcoming this challenge.
Another major advantage to exploiting bacteriocins and phage is their ubiquitous nature. Indeed, phage are generally found wherever the pathogen of interest exists (Gill and Hyman 2010). In terms of bacteriocins, the vast amount of genomic data which is being generated means that in silico-based approaches can be used to identify putative bacteriocins in whole microbial communities.
The food industry is already benefitting from commercially available phage and bacteriocin preparations. However, there is scope for improvement by expanding the range of bacteriocins and phage and exploiting innovations such as nanoencapsulation and antimicrobial packaging which have been shown to improve/sustain antimicrobial efficacy.
In terms of gut health, bacteriocins and phage have the potential to eliminate specific pathogens and or pathobionts (native gut residents with potential to cause disease), and recent research suggests that bacteriocins can potentially shape microbiota composition in favour of human health and could represent viable therapeutics in the treatment and management of diseases. Research into bacteria–phage dynamics in the gut will undoubtedly open up novel ways of exploiting phage to improve health and may even see the use of prophage therapy as a means of stimulating microbial diversity, a key feature of a healthy ecosystem.
While the latter concepts may seem distant aspirations for the moment, the urgent need for narrow-spectrum antimicrobials and the body of evidence supporting the efficacy of bacteriocins and phage suggests that they are well on the way to providing useful adjuncts and alternatives to the broad-spectrum antimicrobial agents currently used in food and in the gut.
Conflict of interest. None declared.