Published online Jul 26, 2017. doi: 10.4252/wjsc.v9.i7.98
Peer-review started: February 2, 2017
First decision: March 31, 2017
Revised: April 27, 2017
Accepted: June 6, 2017
Article in press: June 8, 2017
Published online: July 26, 2017
To establish a hypoxic environment for promoting osteogenesis in rat marrow stromal cells (MSCs) using osteogenic matrix cell sheets (OMCSs).
Rat MSCs were cultured in osteogenic media under one of four varying oxygen conditions: Normoxia (21% O2) for 14 d (NN), normoxia for 7 d followed by hypoxia (5% O2) for 7 d (NH), hypoxia for 7 d followed by normoxia for 7 d (HN), or hypoxia for 14 d (HH). Osteogenesis was evaluated by observing changes in cell morphology and calcium deposition, and by measuring osteocalcin secretion (ELISA) and calcium content. In vivo syngeneic transplantation using OMCSs and β-tricalcium phosphate discs, preconditioned under NN or HN conditions, was also evaluated by histology, calcium content measurements, and real-time quantitative PCR.
In the NN and HN groups, differentiated, cuboidal-shaped cells were readily observed, along with calcium deposits. In the HN group, the levels of secreted osteocalcin increased rapidly from day 10 as compared with the other groups, and plateaued at day 12 (P < 0.05). At day 14, the HN group showed the highest amount of calcium deposition. In vivo, the HN group showed histologically prominent new bone formation, increased calcium deposition, and higher collagen type I messenger RNA expression as compared with the NN group.
The results of this study indicate that modifying oxygen tension is an effective method to enhance the osteogenic ability of MSCs used for OMCSs.
Core tip: Bone tissue engineering using marrow stromal cells (MSCs) is a promising method in regenerative medicine. Here, we have reported a scaffold-free transplantation technique using hypoxic-preconditioned osteogenic matrix cell sheets (OMCSs) derived from MSCs. We show that modifying the oxygen tension before implantation of OMCS composites led to an increased osteogenic capacity of rat bone MSCs.
- Citation: Inagaki Y, Akahane M, Shimizu T, Inoue K, Egawa T, Kira T, Ogawa M, Kawate K, Tanaka Y. Modifying oxygen tension affects bone marrow stromal cell osteogenesis for regenerative medicine. World J Stem Cells 2017; 9(7): 98-106
- URL: https://www.wjgnet.com/1948-0210/full/v9/i7/98.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v9.i7.98
Bone defects resulting from severe fracture, infection, or tumor resection are serious problems that impair patient quality of life. Bone tissue engineering is a novel way to produce sufficient bone to repair bony defects. Various methods[1-5] have examined the use of marrow stromal cells (MSCs) as a cell source for bone tissue engineering, and several reports have incorporated natural or synthetic scaffolds to maintain MSCs[6-8]. However, each method has its limitations, including possible immunological responses against natural materials, and reduced bioactivity or biocompatibility with synthetic materials[9]. So, too, have several recombinant proteins, such as bone morphogenetic protein-2, been vigorously tested in conjunction with these scaffolding materials because of their capacity to enhance osteogenesis. However, the use of recombinant proteins is limited, as it is difficult to control their dosage, with multiple applications or genetic manipulation, such as gene transfer, often required to accommodate their short half-life in vivo. Thus, more effective and simpler methods are needed to enhance osteogenesis for bone tissue reconstruction.
Oxygen tension affects the proliferation and differentiation of MSCs[10,11], and cell culture conditions are generally set to approximately 21% O2 and 5% CO2 at 37 °C. However, several studies have reported that, in the bone marrow, physiological oxygen concentrations range from 6.6% to 8.6% (54.9 mmHg to 71.4 mmHg), as measured using a polarographic needle electrode[12,13]. In a study focusing on the effect of hypoxia on MSC osteogenesis, Lennon et al[10] reported that 5% O2 in primary and subcultures of rat MSCs could enhance osteogenesis. Conversely, D’Ippolito et al[11] have shown that 3% O2 inhibits osteogenic differentiation of human MSCs. Thus, the ideal concentration, duration, and timing of hypoxic treatment to enhance osteogenesis remain unclear.
We previously reported several methods for musculoskeletal reconstruction using cell transplantation techniques using composites of artificial bone combined with MSCs, and the use of a scaffold-free cell sheets, referred to as osteogenic matrix cell sheets (OMCSs). As hypoxic treatment may enhance osteogenesis for such composites and cell sheets, in the present study, we evaluated whether hypoxia could enhance MSCs and the osteogenesis of OMCSs created from MSCs.
All experimental protocols using animals were approved by the Animal Experimental Review Board of the Nara Medical University before experimentation. Animals were housed in a temperature-controlled environment at approximately 21 °C under a 12-h light/12-h dark cycle with free access to food and water.
Seven-week-old male Fisher-344 rats (Japan SLC, Shizuoka, Japan) were purchased for bone marrow cell preparation. Bone marrow cells were obtained from the femoral shafts of rats as previously described[1]. Briefly, both ends of the femurs were cut from the epiphysis, and the marrow was flushed with 10 mL of basal medium expelled from a syringe through a 21-gauge needle. Basal medium consisted of Eagle’s Minimal Essential Medium (Nacalai Tesque, Kyoto, Japan), 15% fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA, United States) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin; Nacalai Tesque). The flushed cells were collected into two T-75 flasks (Falcon, BD Biosciences, Franklin Lakes, NJ, United States) containing 15 mL of basal medium. Non-adherent cells were removed during media changes, which were performed three times per week. Cultures were maintained in a humidified atmosphere of 95% air and 5% CO2 at 37 °C.
After reaching confluence, cells were released by trypsin/EDTA (Nacalai Tesque) and used for in vitro and in vivo experiments.
Osteogenic cell culture and experimental design: Cells were seeded at a density of 1 × 104 cells/cm2 in 12-well cell culture plates (Falcon, BD Biosciences), and subcultured in osteogenic medium consisting of basal medium supplemented with 10 nmol/L dexamethasone (Sigma-Aldrich, St. Louis, MO, United States) and 0.28 mmol/L ascorbic acid phosphate (Wako Pure Chemical Industrials, Kyoto, Japan). These subcultured cells were exposed to one of four variable oxygen concentration conditions: Normoxia (21% O2) for 14 d (NN), normoxia for the first 7 d followed by hypoxia (5% O2) for the next 7 d (NH), hypoxia for the first 7 d followed by normoxia for the next 7 d (HN), and hypoxia for 14 d (HH). The entire experiment was repeated using cells from two different animals. The number of replicates within each assay.
Osteocalcin secretion measurement: Osteocalcin secretion was measured to evaluate the osteogenic potential of MSCs. Secreted osteocalcin is a reliable marker for predicting in vivo osteogenic potential for bone tissue engineering[14]. Secreted osteocalcin levels were measured on days 7, 10, 12, and 14 using an enzyme-linked immunosorbent assay (ELISA) with anti-rat osteocalcin monoclonal antibody (DS Pharma Biomedical, Osaka, Japan). A media change performed 48 h before collection (n = 5 for each group).
Observations of cell morphology and calcium deposition: After 14 d of osteogenic culture, the media was replaced with phosphate-buffered saline (PBS; Gibco, Paisley, United Kingdom), and cell morphology and calcium deposition on the culture plates were observed using an inverted microscope (Eclipse Ti-S, Nikon, Tokyo, Japan). Images were taken using a digital camera (Digital Sight DS-Fi1, Nikon) (n = 5 for each group).
Measurement of calcium deposition: After observations of cell morphology and calcium deposition, total calcium was extracted from each well with 2.0 mL of 20% formic acid, and measured using a methylxylenol blue method (Calcium E-test Wako Kit, Wako Pure Chemical Industrials) (n = 5 for each group). Measurements were adjusted to the total amount of protein in each well, as determined using a spectrocolorimetric method with bovine serum albumin as a standard.
Alkaline phosphatase staining: For alkaline phosphatase (ALP) staining[1], cells were cultured in osteogenic medium for 14 d in 6-well plates under variable oxygen conditions, rinsed twice with PBS, and then stained with naphthol-AS-MX phosphate sodium salt (Sigma-Aldrich) and fast red violet LB salt (Nacalai Tesque) at room temperature for 10 min. The stain was removed by rinsing with tap water, and air dried.
Cell proliferation assay: A colorimetric assay using tetrazolium salt was performed to assess the effect of hypoxic conditions on the proliferative capacity of MSCs. Cells were seeded at a density of 1 × 104 cells/cm2 into the wells of a 96-well cell culture plate (Falcon, BD Biosciences) and subcultured in basal medium for 3 d under hypoxia or normoxia. Cell proliferation was then measured using a cell proliferation assay kit (Promega, Fitchburg, WI, United States), as per the manufacturer’s recommendations (n = 6 for each group).
Syngeneic OMCS transplantation:In vivo syngeneic transplantation experiments were performed using OMCSs prepared from second-passage bone marrow cells, as previously reported[2,3]. OMSCs were exposed to one of two oxygen conditions: Normoxia (21% O2) for 14 d (NN) or hypoxia (5% O2) for the first 7 d followed by normoxia for the next 7 d (HN); these two conditions were chosen based on the results of the in vitro experiments. β-tricalcium phosphate (β-TCP) discs (Hoya, Tokyo, Japan; 75% porosity, 5-mm diameter, and 2-mm thickness) were wrapped with OMCSs and then implanted at subcutaneous sites on the backs of recipient rats.
In this experiment, OMCSs were wrapped around β-TCP discs so that the sheets could be easily identified and harvested for precise histological evaluation.
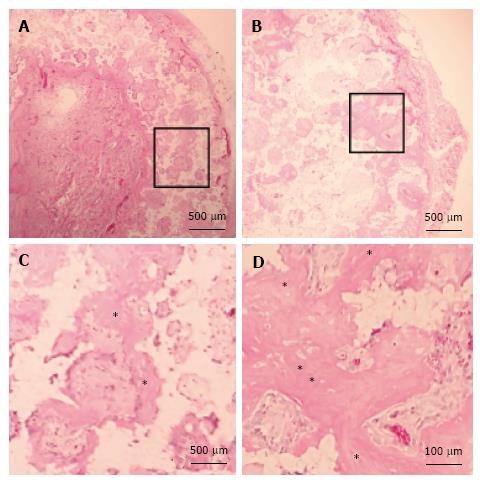
Histological evaluation: β-TCP discs with OMCSs were harvested after 4 wk. Samples were decalcified in K-CX solution (Falma, Tokyo, Japan), embedded in paraffin, cut at the middle of the specimen, and stained with hematoxylin and eosin (HE). Two authors (KI and TE), blinded to the grouping, evaluated the histological findings (n = 5 for each group).
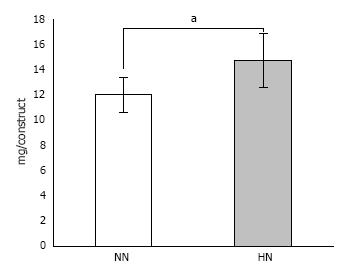
Calcium content measurements: To quantify the amount of newly formed hydroxyapatite, harvested constructs were homogenized, and calcium content was measured as described earlier (n = 5 for each group).
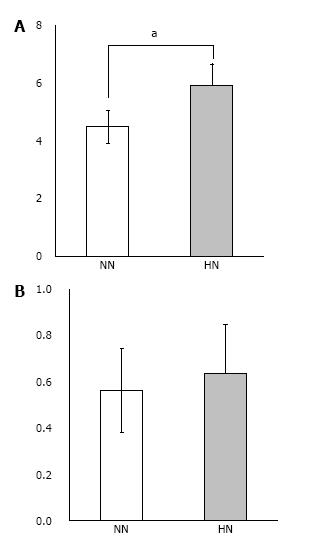
Real-time quantitative PCR: Total RNA was extracted from harvested specimens using ISOGEN (Nippon Gene, Tokyo, Japan), and reverse transcribed into complementary DNA. Real-time quantitative PCR (Applied Biosystems Step One Plus Real Time PCR System, Thermo Fisher Scientific, MA, United States) was performed to measure the expression of collagen type I (Rn00801649 g1) and osteocalcin (Rn01455285 g1) (n = 5 for each group). Thermal cycling conditions were 20 s at 95 °C for activation (TaqMan Fast Universal PCR Master Mix, Thermo Fisher Scientific) followed by 40 cycles of 1 s at 95 °C for denaturation, and 20 s at 60 °C for annealing and extension. Expression levels were normalized to β-actin (Rn00667869 m1). All experiments were performed in duplicate.
Mann-Whitney U-test and one-way ANOVA with Bonferroni post hoc multiple comparisons were performed using SPSS Ver. 17.0 (IBM. Chicago, IL, United States). P values less than 0.05 were considered statistically significant for both tests.
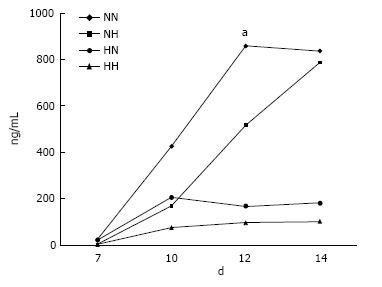
Osteocalcin secretion measurement: At day 7, osteocalcin secretion levels were low in all four groups. In the HN group, levels started to increase rapidly at day 10, peaking and stabilizing from day 12 (P < 0.05). Levels also increased steadily for cells in the NN group, but remained low for cells in the NH and HH groups through day 14 (Figure 1).
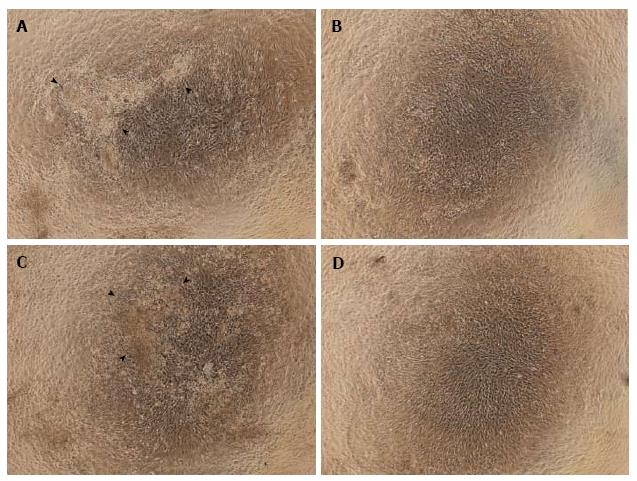
Observations of cell morphology and calcium deposition: In the NN and HN groups, the differentiated cells appeared cuboidal, and cell nodules with calcium deposition were observed. In the NH and HH groups, however, few such nodules were seen. Instead, the cells in the NH and HH groups appeared spindle-shaped and undifferentiated, particularly in the HH group (Figure 2).
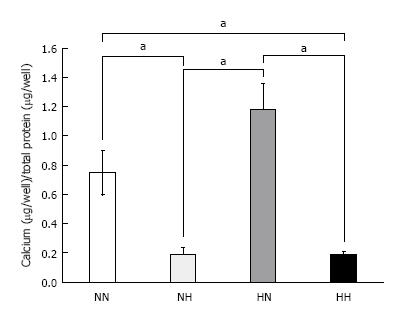
Calcium content measurements: At day 14, we compared the adjusted total calcium deposition in the culture wells among the four groups. The HN group showed the highest calcium deposition, followed by the NN group, as compared with HH and NH groups (P < 0.05) (Figure 3).
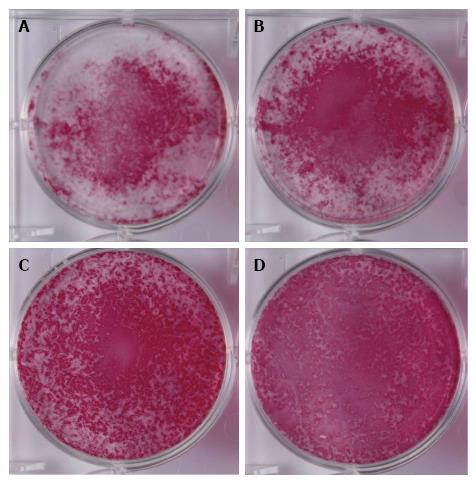
Alkaline phosphatase staining: The NH, HN, and HH groups showed broader positive staining areas as compared with the NN group. Among the three groups, the HN group showed the strongest and the broadest ALP staining. Staining intensity was weakest in the HH group (Figure 4).
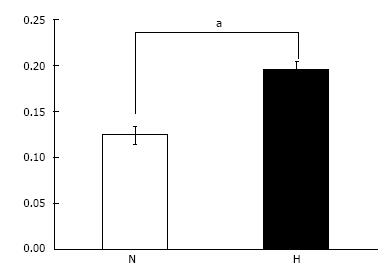
Cell proliferation assay: Cell proliferation for cells grown under hypoxic conditions was significantly higher than that of cells grown under normoxic conditions (P < 0.05) (Figure 5).
Histological evaluation: HN constructs harvested from rats showed prominent new bone formation within the pores of the β-TCP discs. In contrast, there was less new bone formation observed in the NN constructs. This could be due to the reduced osteogenic ability of second-passage MSCs; although, the same cells were used for the HN group. The histological findings were consistent between the two blinded observers (KI and TE) (Figure 6).
Calcium contents measurement: HN constructs showed a significantly higher amount of calcium deposition as compared with the NN constructs (Figure 7).
Real-time quantitative PCR: We measured significantly higher expression of collagen type I mRNA in the HN constructs as compared with the NN constructs (Figure 8). Furthermore, there was a tendency for higher expression of osteocalcin mRNA, although this difference was not statistically significant.
Hypoxic environments affect all facets of cell function, including survival, proliferation, differentiation, migration, and endocrine and paracrine signaling. Hypoxia is mediated through the activity of transcription factors, most notably hypoxia inducible factor (HIF)[15]. Because of the broad range of effects that hypoxia has on cell function, the effects of hypoxia on the osteogenic ability of MSCs has been controversial[10,11]. In addition, differences in the experimental cell types and culture methods, and the timing of exposure to the hypoxic environment can lead to variation in findings among different studies. On the basis of our methods for regenerative medicine, we show that hypoxia (5% O2) enhances cell proliferation when delivered for 7 d followed by normoxia for 7 d (HN group), with observed increases in osteocalcin secretion, calcium deposition, and ALP staining, suggestive of enhanced osteogenesis in MSCs. The results of our in vivo experiments also show that hypoxic pretreatment can drive higher rates of osteogenesis as compared with normal oxygen levels.
With regards to cell survival, others[16] have shown that hypoxic preconditioning (0.5% O2 for 24 h) both in vitro and in vivo can increase the expression of pro-survival factors, Bcl-2 and Bcl-xL, and reduce cell death and caspase-3 activation in stem cells as compared with stem cells grown under normal oxygen conditions. Despite some contradictory results[17,18], many other studies also support the positive effects of hypoxia (1.5%-8% O2) on MSC proliferation[10,11,19-22]. Here, we also showed enhanced MSC proliferation with hypoxic preconditioning, and suggest that the elevated osteogenic ability of the cells in the HN group may have arisen from the enhanced cell proliferation during the first 7 d of hypoxic culture.
D’Ippolito et al[11] previously reported an inhibitory effect of hypoxia on osteogenic differentiation of human marrow-isolated adult multilineage-inducible cells. However, they also reported that hypoxia shortened population doubling time, resulting in an increase in the rate of cell proliferation, which agrees with our results. However, in their study, hypoxia was employed throughout the culture period. In our study, we found that cells treated continuously under hypoxic conditions (HH group; 5% hypoxia for 14 d) showed lower proliferation, less osteogenic ability, and retained an undifferentiated morphology. Collectively, these data suggest that the appropriate timing of hypoxia is important for stimulating osteogenesis. To the best of our knowledge, our study is the first to seek the best temporal combination of hypoxia and normoxia during osteogenic culture.
Environmental preconditioning, such as through the use of hypoxia or growth factor supplementation offers a potentially powerful approach to enhance the proliferative and differentiative ability of MSCs[23]. Hagmann et al[24] added fibroblast growth factor-2 during human MSCs expansion, and found higher cell population growth indices and a downregulation of CD146, a marker of endothelial cells. Others have shown that decellularized extracellular matrix (dECM), deposited by stem cells, is another promising approach to create environmental preconditioning. He et al[25] construct a dECM by culturing porcine synovium-derived stem cells (SDSCs) on fibronectin-coated surfaces, and then lysed the cells using a nonionic surfactant. The authors then seeded SDSCs onto the dECM and found increased cell numbers and enhanced chondrogenic capacity as compared with cells seeded on control substrates.
Numerous studies have reported the use of bioengineered scaffolds for enhanced bone repair[6-8]. However, considering the potential disadvantages of scaffolds, such as reduced biocompatibility[9] or the possibility of causing an immunological response, we believe that scaffold- and recombinant protein-free techniques are better solutions. Recently, we reported the culture of MSCs as OMCSs, and demonstrated their osteogenic potential in vitro and bone formation in vivo in the absence of scaffolds[2]. Furthermore, OMCSs can be used in non-union surgery and to accelerate bone-tendon healing in ligament reconstruction[3]. Here, we showed that OMCSs preconditioned with hypoxic conditions offers a simple and inexpensive method to improve the osteogenic capacity of cells, and this may provide a better method for MSC-mediated bone tissue engineering.
Hypoxia as an environment preconditioning tool has been explored predominantly as a strategy to enhance the chondrogenic ability of MSCs[23]. Human MSCs, preconditioned under hypoxic and normoxic environments, were encapsulated in alginate hydrogels and implanted subcutaneously onto the backs of nude mice. Whereas the hypoxia-preconditioned implants retained a chondral phenotype after implantation, the normoxia-preconditioned implants underwent calcification, vascular invasion, and subsequent endochondral ossification[26]. Jukes et al[27] also utilized endochondral ossification to engineer bone tissue from mouse embryonic stem cells; however, the authors did not modulate the oxygen tension. We speculate that the increased osteogenic ability of the cells in the HN group in our study also developed through endochondral ossification. However, the time course is relatively short and additional experiments will be needed to confirm this hypothesis.
There were a few limitations in this study. First, because we used only rat MSCs, evaluations using human MSCs should be performed in future experiments. Second, more detailed intracellular signaling changes caused by the hypoxic environment should be elucidated at the molecular level. Third, although the temporal combination of hypoxia and normoxia clearly boosted osteogenesis of rat MSCs, it is unclear whether this increased osteogenesis is solely caused by the increase in cell number or whether hypoxic preconditioning also enhanced the differentiation and mineralization of these cells. Despite these limitations, our findings offer an important insight into the potential for hypoxic preconditioning in the field of bone regenerative medicine.
In conclusion, we show that modifying oxygen tension can improve the higher osteogenic ability of rat MSCs both under in vitro and in vivo conditions. Thus, hypoxic preconditioning appears to be an effective method for MSC-mediated bone tissue engineering.
We would like to thank to Ms. F. Kunda and Ms. M. Matsumura (Nara Medical University) for their technical assistance.
Bone tissue engineering using marrow stromal cells (MSCs) as a cell source has been widely studied. Among several factors affecting MSC osteogenesis, oxygen tension is important; however, the specific tension and timing of hypoxic preconditioning remains controversial. Here, the authors investigated how hypoxia affects the osteogenic ability of rat MSCs in vitro and within cell sheets in a subcutaneous scaffold in vivo.
Various methods have been reported to enhance the osteogenic ability of MSCs, including the use of scaffolds and growth factors. However, considering the potential disadvantages of these approaches, such as immunological responses to the scaffold, as well as the complicated procedure of producing suitable scaffolds for insertion, techniques that avoid these approaches would be better suited. In this context, the authors suggest that modulating oxygen tension and the use of osteogenic matrix cell sheet (OMCS) could offer a promising method for bone repair.
Here, the authors show that modifying oxygen tension using hypoxia preconditioning can enhance the osteogenic ability of MSCs in vitro, and similarly advance bone formation in OMCS wrapped around scaffolds when implanted subcutaneously on the backs of rats.
Modulating oxygen tension to enhance osteogenesis is a simple and inexpensive preconditioning method. Combining OMCSs with appropriately timed hypoxia can enhance bone tissue engineering.
MSCs: Marrow stromal cells, derived from the bone marrow. MSCs can be induced to differentiate into osteogenic, chondrogenic, adipogenic or other cell lineages with the appropriate media conditions. These cells are routinely used as a cell source for musculoskeletal tissue engineering purposes. OMSCs: Osteogenic matrix cell sheets are MSCs cultured with dexamethasone and ascorbic acid phosphate (originally with also β-glycerophosphate). The cells undergo differentiation and matrix production, producing a cell sheet structure that can be collected as a single cell sheet. These sheets offer in vitro osteogenic potential and in vivo bone formation without the need for scaffolds. OMSCs in the current study were implanted into the backs of rats using a β-TCP scaffold for positioning and to be able to identify the OMSC later for harvesting to measure bone formation changes. OMCSs can be used alone for non-union surgery and to accelerate bone-tendon healing in ligament reconstruction.
This study is new. This manuscript aims at investigating whether modifying oxygen tension affected MSC osteogenesis. The authors found that low oxygen pretreatment for 7-d following by 7-d treatment under normal oxygen could promote MSCs’ osteogenic differentiation in both in vitro and in vivo models.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Pei M S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Ohgushi H, Dohi Y, Katuda T, Tamai S, Tabata S, Suwa Y. In vitro bone formation by rat marrow cell culture. J Biomed Mater Res. 1996;32:333-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Akahane M, Nakamura A, Ohgushi H, Shigematsu H, Dohi Y, Takakura Y. Osteogenic matrix sheet-cell transplantation using osteoblastic cell sheet resulted in bone formation without scaffold at an ectopic site. J Tissue Eng Regen Med. 2008;2:196-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Inagaki Y, Uematsu K, Akahane M, Morita Y, Ogawa M, Ueha T, Shimizu T, Kura T, Kawate K, Tanaka Y. Osteogenic matrix cell sheet transplantation enhances early tendon graft to bone tunnel healing in rabbits. Biomed Res Int. 2013;2013:842192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641-650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3539] [Cited by in F6Publishing: 3155] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 5. | Ueha T, Akahane M, Shimizu T, Uchihara Y, Morita Y, Nitta N, Kido A, Inagaki Y, Kawate K, Tanaka Y. Utility of tricalcium phosphate and osteogenic matrix cell sheet constructs for bone defect reconstruction. World J Stem Cells. 2015;7:873-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Chiu LH, Lai WF, Chang SF, Wong CC, Fan CY, Fang CL, Tsai YH. The effect of type II collagen on MSC osteogenic differentiation and bone defect repair. Biomaterials. 2014;35:2680-2691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | He Y, Dong Y, Cui F, Chen X, Lin R. Ectopic osteogenesis and scaffold biodegradation of nano-hydroxyapatite-chitosan in a rat model. PLoS One. 2015;10:e0135366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Qi Y, Niu L, Zhao T, Shi Z, Di T, Feng G, Li J, Huang Z. Combining mesenchymal stem cell sheets with platelet-rich plasma gel/calcium phosphate particles: a novel strategy to promote bone regeneration. Stem Cell Res Ther. 2015;6:256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Elloumi-Hannachi I, Yamato M, Okano T. Cell sheet engineering: a unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J Intern Med. 2010;267:54-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 10. | Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 330] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | D’Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 12. | Harrison JS, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99:394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Maurer P, Meyer L, Eckert AW, Berginski M, Schubert J. Measurement of oxygen partial pressure in the mandibular bone using a polarographic fine needle probe. Int J Oral Maxillofac Surg. 2006;35:231-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Nakamura A, Dohi Y, Akahane M, Ohgushi H, Nakajima H, Funaoka H, Takakura Y. Osteocalcin secretion as an early marker of in vitro osteogenic differentiation of rat mesenchymal stem cells. Tissue Eng Part C Methods. 2009;15:169-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Guillemin K, Krasnow MA. The hypoxic response: huffing and HIFing. Cell. 1997;89:9-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 16. | Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 456] [Cited by in F6Publishing: 443] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 17. | Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 171] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, Prockop DJ. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007;2:e416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 19. | Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207:331-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 321] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 20. | Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948-953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 377] [Cited by in F6Publishing: 377] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 21. | Ren H, Cao Y, Zhao Q, Li J, Zhou C, Liao L, Jia M, Zhao Q, Cai H, Han ZC. Proliferation and differentiation of bone marrow stromal cells under hypoxic conditions. Biochem Biophys Res Commun. 2006;347:12-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Carrancio S, López-Holgado N, Sánchez-Guijo FM, Villarón E, Barbado V, Tabera S, Díez-Campelo M, Blanco J, San Miguel JF, Del Cañizo MC. Optimization of mesenchymal stem cell expansion procedures by cell separation and culture conditions modification. Exp Hematol. 2008;36:1014-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | Pei M. Environmental preconditioning rejuvenates adult stem cells’ proliferation and chondrogenic potential. Biomaterials. 2017;117:10-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Hagmann S, Moradi B, Frank S, Dreher T, Kämmerer PW, Richter W, Gotterbarm T. FGF-2 addition during expansion of human bone marrow-derived stromal cells alters MSC surface marker distribution and chondrogenic differentiation potential. Cell Prolif. 2013;46:396-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | He F, Chen X, Pei M. Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15:3809-3821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Leijten J, Georgi N, Moreira Teixeira L, van Blitterswijk CA, Post JN, Karperien M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc Natl Acad Sci USA. 2014;111:13954-13959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Jukes JM, Both SK, Leusink A, Sterk LM, van Blitterswijk CA, de Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:6840-6845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |