Abstract
In order to investigate chemical evolution in interstellar molecular clouds, enantiomer-selective photo-induced chemical reactions between an amino acid and disaccharides in the gas phase were examined using a tandem mass spectrometer containing an electrospray ionization source and a cold ion trap. Ultraviolet photodissociation mass spectra of cold gas-phase noncovalent complexes of protonated tryptophan (Trp) enantiomers with disaccharides consisting of two d-glucose units, such as d-maltose or d-cellobiose, were obtained by photoexcitation of the indole ring of Trp. NH2CHCOOH loss via cleavage of the Cα–Cβ bond in Trp induced by hydrogen atom transfer from the NH3 + group of a protonated Trp was observed in a noncovalent heterochiral H+(l-Trp)(d-maltose) complex. In contrast, a photo-induced chemical reaction forming the product ion with m/z 282 occurs in homochiral H+(d-Trp)(d-maltose). For d-cellobiose, both NH2CHCOOH elimination and the m/z 282 product ion were observed, and no enantiomer-selective phenomena occurred. The m/z 282 product ion indicates that the photo-induced C-glycosylation, which links d-glucose residues to the indole moiety of Trp via a C–C bond, can occur in cold gas-phase noncovalent complexes, and its enantiomer-selectivity depends on the structure of the disaccharide.
Similar content being viewed by others
Introduction
Biomolecules have the ability to distinguish between enantiomers of chiral molecules. For example, one enantiomer of a chiral drug may be highly toxic to the human biological system, while the other may be medically effective. Therefore, determination of the enantiomeric excess of chiral drugs is vital to ensure their safety and efficacy (Srinivas 2004; McConnell et al. 2007). Chiral recognition in living systems is attributed to homochirality in biomolecules consisting of l-amino acids and d-sugars. The origin of homochirality in biomolecules is one of the most interesting fields of scientific research (Bonner 1991).
Analysis of amino acids and dipeptides found in models of interstellar ice suggests the abiotic formation of biological molecules under extraterrestrial conditions (Bernstein et al. 2002; Muñoz Caro et al. 2002; Gontareva et al. 2009; Kaiser et al. 2013; Abplanalp et al. 2016). However, no enantiomeric enrichment has been observed. Excess l-amino acids found in the Murchison meteorite (Cronin and Pizzarello 1997; Engel and Macko 1997; Pizzarello and Groy 2011) and the discovery of the interstellar chiral molecule propylene oxide (McGuire et al. 2016) imply an extraterrestrial origin of enantiomeric enrichment. Circular polarization of light in star-formation regions suggests the possibility of enantioselective photodissociaton, in which circularly polarized light induces the formation of excess l-amino acids in interstellar space (Bailey et al. 1998; Meinert et al. 2011). A hypothesis for the extraterrestrial origin of biomolecules has been proposed on the basis of these studies, according to which abiotic formation of racemic amino acids occurs in interstellar molecular clouds, followed by enantioselective photodissociation of d-enantiomers in the presence of circularly polarized light.
Chemical reactions in interstellar molecular clouds occur at low temperatures and low densities. We investigated the structure and reactivity of mass-selected and temperature-controlled gas-phase noncovalent complexes containing biological molecules, as a model of interstellar molecular clouds. Above 170 K, the photo-induced Cα–Cβ bond cleavage of cold protonated d-tryptophan H+(d-Trp) on a chiral crown ether in the gas phase was suppressed with increasing temperatures. At 300 K, no difference in reactivity was observed between the d- and l-enantiomers (Fujihara et al. 2014a, 2015a). The temperatures of the gas-phase noncovalent complexes correspond to those of interstellar and atmospheric molecular clouds. When three l-serine molecules are present in a protonated cluster, photo-induced dissociation of Trp in the cluster is enantiomer-selective (Fujihara et al. 2014b). Enantiomer-selective collision-activated dissociation of gas-phase Trp is induced through protonation due to chiral recognition by the alanine tripeptide (Fujihara et al. 2017a). The chiral recognition ability of alanine peptides in the gas phase was analyzed by photodissociation experiments for various peptide sizes (Fujihara et al. 2016). The enantiomeric selectivity of amino acids and peptides in gas-phase clusters depended on the cluster size. The enantiomer-selective photodissociation of cold gas-phase Trp via chiral recognition was used for the enantiomeric excess determination of biological molecules in solution (Fujihara et al. 2015a, 2015b, 2016, 2017b; Fujihara and Maeda 2017). Photodissociation of cold gas-phase noncovalent complexes of H+(l-Trp) with monosaccharides showed that l-Trp dissociated upon noncovalent complexation with d-glucose (d-Glc) or d-galactose (d-Gal) (Fujihara et al. 2017b). The results suggested that d-monosaccharides induced the formation of excess d-amino acids via enantiomer-selective photodissociation in interstellar molecular clouds, and are not consistent with the homochirality in biomolecules consisting of l-amino acids and d-sugars.
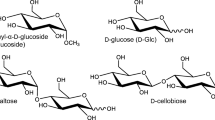
In this study, we carried out ultraviolet photoexcitation of cold gas-phase noncovalent complexes of protonated Trp enantiomers and disaccharides. The chemical reaction between protonated Trp and disaccharides such as d-maltose and d-cellobiose was analyzed for chiral recognition and enantiomeric selectivity. d-Maltose and d-cellobiose are disaccharides consisting of two d-Glc units linked by α1,4 and β1,4 bonds, respectively. Figure 1 shows the structures of the molecules. On the basis of the results, we discussed the relationship between enantiomer-selective chemical reactions and chemical evolution in interstellar molecular clouds.
Methods
l-Trp, d-Trp, d-maltose, and d-cellobiose were obtained from Nacalai Tesque. A solution containing 0.5 mM of Trp and 1.0 mM of a disaccharide in a mixture of water and methanol (50/50, v/v) with 1% acetic acid was used. Details of the home-built tandem mass spectrometer containing an electrospray ionization source and a variable-temperature 22-pole ion trap have been described previously (Fujihara et al. 2014a). Noncovalent gas-phase complexes of protonated Trp enantiomers with disaccharides were generated using electrospray ionization, and then mass-selected using a quadrupole mass filter. The mass-selected ions were temperature-controlled at 8 K using a cold ion trap, in which the trapped ions were thermalized by multiple collisions with a helium buffer gas, analogous to a thermal bath. The mass-selected and temperature-controlled ions were extracted from the trap and then irradiated with a photodissociation laser pulse. The fourth harmonic of a Nd:YAG laser (Minilite II, Continuum) was used as the photodissociation light. The wavelength was 266 nm, and the energy was approximately 1.0 mJ/pulse. The fragment ions were mass-analyzed using a reflectron time-of-flight mass spectrometer.
Results and Discussion
Figure 2 shows the photodissociation mass spectra of heterochiral H+(l-Trp)(d-maltose) and homochiral H+(d-Trp)(d-maltose) (m/z 547) at 8 K. The irradiation wavelength was 266 nm, which excites the indole ring of Trp to the ππ* state. Protonated Trp (m/z 205) formed by evaporation of d-maltose is the main product ion for both heterochiral and homochiral noncovalent complexes. The spectra are normalized using an ion intensity of 100 for the protonated Trp. The fragment ion m/z 188 observed in both spectra corresponds to the NH3-elimination product of free protonated Trp. NH3 loss from free protonated Trp is its primary dissociation pathway (Lioe and O’Hair 2004; Aribi et al. 2004). The proton remains associated with Trp in the noncovalent complexes, and the protonated Trp has both NH3 + and COOH groups.
Photodissociation mass spectra of (a) H+(l-Trp)(d-maltose) and (b) H+(d-Trp)(d-maltose) (m/z 547) at 8 K. The irradiation wavelength was 266 nm, which excites the indole ring of Trp to the ππ* state. The spectra are normalized using an ion intensity of 100 for protonated Trp (m/z 205) formed by evaporation of d-maltose
NH2CHCOOH loss from the noncovalent complexes is observed at m/z 473, and the NH2CHCOOH-elimination product ion intensity of heterochiral H+(l-Trp)(d-maltose) is larger than that of homochiral H+(d-Trp)(d-maltose). NH2CHCOOH loss from the heterochiral complex represents the enantiomer-selective photodissociation of protonated Trp via Cα–Cβ bond cleavage induced by chiral recognition by d-maltose. The Cα–Cβ bond cleavage of Trp in the gas phase is also observed in the photodissociation mass spectra of heterochiral noncovalent complexes of protonated Trp with a chiral crown ether or monosaccharides (Fujihara et al. 2014a, 2015a, 2017b). It has been reported that enantiomer-selective photodissociation via Cα–Cβ bond cleavage is induced by hydrogen atom transfer from the NH3 + group of protonated Trp in the heterochiral noncovalent complexes, whereas dissociation of Trp in the homochiral complexes is suppressed by energy release through the evaporation of molecules.
In the spectrum of homochiral H+(d-Trp)(d-maltose) shown in Fig. 2b, a product ion is observed at m/z 282 in addition to that of H+(d-Trp) (m/z 205). The m/z 282 product ion is not formed by the photoexcitation of heterochiral H+(l-Trp)(d-maltose), as shown in Fig. 2a. This indicates that the enantiomer-selective photodissociation that forms the m/z 282 product ion only occurs in homochiral H+(d-Trp)(d-maltose). The m/z 282 product ion is not formed with monosaccharides such as Glc and Gal (Fujihara et al. 2017b), and cannot be explained by fragmentation alone. Thus, a photo-induced chemical reaction between H+(d-Trp) and d-maltose occurs in the gas phase, and this reaction is enantiomer-selective.
Photoexcitation experiments with cold gas-phase noncovalent complexes of protonated Trp enantiomers with d-cellobiose were performed to further investigate the enantiomer-selective photo-induced chemical reaction. d-Cellobiose is a disaccharide consisting of two d-Glc units linked by a β1,4 bond, whereas d-maltose is formed from two d-Glc units joined by a α1,4 bond, as shown in Fig. 1. Figure 3 shows the photodissociation mass spectra of heterochiral H+(l-Trp)(d-cellobiose) and homochiral H+(d-Trp)(d-cellobiose) (m/z 547) at 8 K. Protonated Trp (m/z 205) formed by evaporation of d-cellobiose is the main product ion, as for the case with d-maltose. NH2CHCOOH loss from the noncovalent complexes (m/z 473) and the m/z 282 product ion are observed in both the heterochiral and homochiral noncovalent complexes. No difference in reactivity between the Trp enantiomers is observed in the spectra, and thus enantiomer-selective phenomenon are not observed in the photoexcitation of protonated Trp enantiomers with d-cellobiose.
The m/z 282 product ion is formed by the photoexcitation of cold gas-phase protonated Trp with disaccharides. This chemical reaction is enantiomer-selective in the case with d-maltose. The glycosidic bond cleavage and C–C bond formation linking a saccharide to a Trp residue in the noncovalent complexes could be considered to rationalize the m/z 282 product ion, because the product cannot be explained by fragmentation alone. Figure 4 shows a proposed structure for the m/z 282 product ion optimized by theoretical calculations at the B3LYP/6–311++G(d,p) level using GAUSSIAN 09 (Frisch et al. 2009). A d-Glc residue is linked via a C–C bond to the C2 atom of the indole moiety. A proton attaches to the N1 atom of the indole ring. It has been reported that ultraviolet photoexcitation of gas-phase protonated Trp induces electron transfer from the indole ring to the NH3 + group, which forms hydrogen atom (Grégoire et al. 2009; Pérot et al. 2010). The positive charge resulted from the electron transfer localizes the C2 atom of the indole moiety. The proposed structure is also based on that of C-glycosyl tryptophans, such as C-mannosylated tryptophans, which have been identified in various proteins (Hofsteenge et al. 1994; Krieg et al. 1998; Doucey et al. 1999; Hartmann and Hofsteenge 2000; Garcia et al. 2000). Glycosylation affects biological activities by influencing higher order structure, and is one of the most important post-translational modifications of proteins. Thus, the photo-induced chemical reaction forming the m/z 282 product ion is assignable to C-glycosylation. The hydrogen atom formed by photoexcitation, which induces the Cα–Cβ bond cleavage of Trp in the noncovalent complexes, can play an important role in the photo-induced chemical reaction. The m/z 282 product ion indicates that enantiomer-selective photo-induced C-glycosylation can occur in cold gas-phase noncovalent complexes, and its enantiomer-selectivity depends on the structure of the disaccharide.
Conclusions
Photodissociation mass spectra of cold gas-phase noncovalent complexes of protonated Trp enantiomers with disaccharides consisting of two d-Glc units were obtained by the photoexcitation of Trp. NH2CHCOOH loss via Cα–Cβ bond cleavage of Trp induced by hydrogen atom transfer was observed in heterochiral H+(l-Trp)(d-maltose), whereas a photo-induced chemical reaction forming the m/z 282 product ion occurs in homochiral H+(d-Trp)(d-maltose). The m/z 282 product ion indicates that photo-induced C-glycosylation, which links a Glc residue to the indole moiety of Trp via a C–C bond, can occur in cold gas-phase noncovalent complexes.
The structures determined by intra- and inter-molecular hydrogen bonds in gas-phase noncovalent complexes play an important role in enantiomer-selective C–C bond formation. To understand chemical evolution in interstellar molecular clouds, it is necessary to determine the structure and reactivity of mass-selected and temperature-controlled gas-phase noncovalent complexes as a model for interstellar molecular clouds using photodissociation spectroscopy with wavelength-variable laser systems and direct comparisons with theoretical calculations.
References
Abplanalp MJ, Förestel M, Kaiser RI (2016) Exploiting single photon vacuum ultraviolet photoionization to unravel the synthesis of complex organic molecules in interstellar ices. Chem Phys Lett 644:79–98
Aribi HE, Orlova G, Hopkinson AC, Siu KWM (2004) Gas-phase fragmentation reactions of protonated aromatic amino acids: concomitant and consecutive neutral eliminations and radical cation formations. J Phys Chem A 108:3844–3854
Bailey J, Chrysostomou A, Hough JH, Gledhill TM, McCall A, Clark S, Ménard F, Tamura M (1998) Circular polarization in star-formation regions: implications for biomolecular homochirality. Science 281:672–674
Bernstein MP, Dworkin JP, Sandford SA, Cooper GW, Allamandola LJ (2002) Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 416:401–403
Bonner WA (1991) The origin and amplification of biomolecular chirality. Orig Life Evol Biosph 21:59–111
Cronin JR, Pizzarello S (1997) Enantiomeric excesses in meteoritic amino acids. Science 275:951–955
Doucey MA, Hess D, Blommers MJJ, Hofsteenge J (1999) Recombinant human interleukin-12 is the second example of a C-mannosylated protein. Glycobiology 9:435–441
Engel MH, Macko SA (1997) Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite. Nature 389:265–268
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, J Hasegawa, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.1. Gaussian, Inc., Wallingford CT
Fujihara A, Maeda N (2017) Quantitative chiral analysis of amino acids in solution using enantiomer-selective photodissociation of cold gas-phase tryptophan via chiral recognition. Anal Chim Acta 979:31–35
Fujihara A, Sato T, Hayakawa S (2014a) Enantiomer-selective ultraviolet photolysis of temperature-controlled protonated tryptophan on a chiral crown ether in the gas phase. Chem Phys Lett 610-611:228–233
Fujihara A, Maeda N, Hayakawa S (2014b) Enantiomer-selective photolysis of cold gas-phase tryptophan in L-serine clusters with linearly polarized light. Orig Life Evol Biosph 44:67–73
Fujihara A, Maeda N, Hayakawa S (2015a) Quantitative chiral analysis of tryptophan using enantiomer-selective photolysis of cold non-covalent complexes in the gas phase. J Mass Spectrom 50:451–453
Fujihara A, Maeda N, Hayakawa S (2015b) Enantioselective photolysis and quantitative chiral analysis of tryptophan complexed with alkali-metalized L-serine in the gas phase. Chirality 27:349–352
Fujihara A, Maeda N, Hayakawa S (2016) Chiral recognition between L-alanine peptides and tryptophan enantiomers probed by ultraviolet photodissociation in the gas phase. J Mass Spectrom 51:257–260
Fujihara A, Matsuyama H, Tajiri M, Wada Y, Hayakawa S (2017a) Enantioselective collision-activated dissociation of gas-phase tryptophan induced by chiral recognition of protonated l-alanine peptides. Orig Life Evol Biosph 47:161–167
Fujihara A, Maeda N, Doan TN, Hayakawa S (2017b) Enantiomeric excess determination for monosaccharides using chiral transmission to cold gas-phase tryptophan in ultraviolet photodissociation. J Am Soc Mass Spectrom 28:224–228
Garcia A, Lenis LA, Jiménez C, Debitus C, Quiñoá E, Riguera R (2000) The occurrence of the human glycoconjugate C 2-α-d-mannosylpyranosyl-l-tryptophan in marine ascidians. Org Lett 2:2765–2767
Gontareva NB, Kuzicheva EA, Shelegedin VN (2009) Synthesis and characterization of peptides after high-energy impact on the icy matrix: preliminary step for further UV-induced formation. Plan Space Sci 57:441–445
Grégoire G, Lucas B, Barat M, Fayeton JA, Dedonder-Lardeux C, Jouvet C (2009) UV photoinduced dynamics in protonated aromatic amino acid. Eur Phys J D 51:109–116
Hartmann S, Hofsteenge J (2000) Properdin, the positive regulator of complement, is highly C-mannosylated. J Biol Chem 275:28569–28574
Hofsteenge J, Müller DR, Beer T, Löffler A, Richter WJ, Vliegenthart JFG (1994) New type of linkage between a carbohydrate and a protein: C-Glycosylation of a specific tryptophan residue in human RNase Us. Biochemistry 33:13524–13530
Kaiser RI, Stockton AM, Kim YS, Jensen EC, Mathies RA (2013) On the formation of dipeptides in interstellar model ices. Astrophys J 765:111–119
Krieg J, Hartmann S, Vicentini A, Wolfgang Gläsner W, Hess D, Hofsteenge J (1998) Recognition signal for C-mannosylation of Trp-7 in RNase 2 consists of sequence Trp-x-x-Trp. Mol Biol Cell 9:301–309
Lioe H, O’Hair RAJ (2004) Gas-phase reactions of protonated tryptophan. J Am Soc Mass Spectrom 15:65–76
McConnell O, Bach A, Balibar C, Byrne N, Cai Y, Carter G, Chlenov M, Di L, Fan K, Goljer I, He Y, Herold D, Kagan M, Kerns E, Koehn F, Kraml C, Marathias V, Marquez B, McDonald L, Nogle L, Petucci C, Schlingmann G, Tawa G, Tischler M, Williamson RT, Sutherland A, Watts W, Young M, Zhang MY, Zhang Y, Zhou D, Ho D (2007) Enantiomeric separation and determination of absolute stereochemistry of asymmetric molecules in drug discovery—building chiral technology toolboxes. Chirality 19:658–682
McGuire BA, Carroll PB, Loomis RA, Finneran IA, Jewell PR, Remijan AJ, Blake GA (2016) Discovery of the interstellar chiral molecule propylene oxide (CH3CHCH2O). Science 352:1449–1452
Meinert C, Pd M, d’Hendecourt LLS, Nahon L, Jones NC, Hoffmann SV, Bredehöft JH, Meierhenrich UJ (2011) Photochirogenesis: photochemical models on the absolute asymmetric formation of amino acids in interstellar space. Phys Life Rev 8:307–330
Muñoz Caro GM, Meierhenrich UJ, Schutte WA, Barbier B, Segovia AA, Rosenbauer H, Thiemann WHP, Brack A, Greenberg JM (2002) Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 416:403–406
Pérot M, Lucas B, Barat M, Fayeton JA, Jouvet C (2010) Mechanisms of UV photodissociation of small protonated peptides. J Phys Chem 114:3147–3156
Pizzarello S, Groy TL (2011) Molecular asymmetry in extraterrestrial organic chemistry: an analytical perspective. Geochim Cosmochim Acta 75:645–656
Srinivas NR (2004) Evaluation of experimental strategies for the development of chiral chromatographic methods based on diastereomer formation. Biomed Chromatogr 18:207–233
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 17 K14441.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doan, T.N., Fujihara, A. Enantiomer-Selective Photo-Induced Reaction of Protonated Tryptophan with Disaccharides in the Gas Phase. Orig Life Evol Biosph 48, 123–130 (2018). https://doi.org/10.1007/s11084-017-9544-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-017-9544-3