Abstract
Hyperphosphatemic familial tumoral calcinosis (HFTC) is a rare, autosomal recessive genetic disease. This disease is characterized by the progressive calcification of soft tissues leading to symptoms of pressure and hyperphosphatemia but normal concentrations of serum calcium with or without an elevation of 1,25-dihydroxyvitamin D3 levels.HFTC is caused by loss-of-function mutations in the GALNT3, FGF23 or KL genes. Here, we identified two novel mutations in the GALNT3 gene in a Chinese family with HFTC. Identification of a novel genotype in HFTC provides clues for understanding the phenotype–genotype relationships in HFTC and may assist not only in the clinical diagnosis of HFTC but also in the interpretation of the genetic information used for prenatal diagnosis and genetic counseling.
Similar content being viewed by others
Introduction
Familial tumoral calcinosis (FTC) is a rare, autosomal recessive genetic disease. The main clinical manifestation of FTC is the development of calcified masses predominantly at peri-articular locations, especially the hips, leading to intolerable pain, skin ulcerations and secondary skin and bone infections.1
According to serum phosphate concentrations, FTC can be classified as hyperphosphatemic (HFTC) or normophosphatemic FTC.2 FTC is caused by a loss-of-function mutation in one of the following genes: FGF23, which encodes the potent phosphaturic protein fibroblast growth factor 23; GALNT3, which encodes the uridine diphosphate-N-acetyl-α-D-galactosamine-polypeptide N-acetylgalactosaminyl-transferase 3 (GalNAc-T3), a glycosyl transferase responsible for FGF23 O-glycosylation and proper secretion; or KL, which encodes Klotho, an FGF23 co-factor required to activate FGF receptors.3–7
The incidences of FTC in men and women are similar; however, the incidence in black patients of African descent is relatively high, followed by that in white patients from the Middle East.1 Only one observed individual with HFTC caused by an FGF23 mutation was Japanese.7,8 Herein, we report two HFTC cases with two novel mutations in the GALNT3 gene.
Materials and methods
Case report
The proband of this study was a 13-year-old boy. When he was 2 years old, his parents accidentally discovered hard masses with slight tenderness on his right hip and feet. With increasing age, the masses increased in size. Several operations were performed to remove the masses on his feet and right hip.
The patient’s sister also had similar masses on her hip (Figure 1) and feet; these masses were excised but recurred post-operatively. No ectopic calcification foci were identified in their parents. The serum phosphate concentrations of the father and mother were normal (Table 1). Both parents were healthy and did not have a history of autoimmune diseases or similar disorders. There was no consanguineous marriage or white or black genetic admixture in this family for the past three generations.
Upon physical examination, the proband was conscious and cooperative. Claudication of the right leg was observed. A large, hard mass with slight tenderness was found on the right hip (Figure 2a). The serum phosphate level for this patient was as high as 2.88 mmol·L−1 (normal range: 0.8–1.6 mmol·L−1). His serum levels of parathyroid hormone (PTH) and 25-hydroxyvitamin D3 were normal.
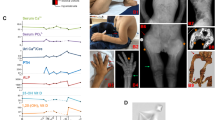
Imaging data of the patient. (a) On the right side of the buttocks of the patient, a 14-cm surgical scar with varicosity of the superficial veins in the region was observed in the region of the abnormal mass that was approximately 18 cm in length. (b) A computed tomography (CT) reconstruction film of the pelvis shows a calcified soft tissue mass in the right hip. Femur: normal bone density. (c) In a T1-weighted magnetic resonance imaging of the pelvis, low intensity signals were obtained at the site of the lesion, indicating the presence of a soft tissue mass in the right hip. (d) A 16×3×5 cm tumor specimen was obtained during the operation; the tumor section showed multiple lobes and a gray allantoic fluid. The size of the cyst within the tumor was 1.5×2.5 cm, and the cyst was encapsulated with a 0.5-cm-thick smooth wall and contained a lime-like material. (e) Histological analysis showed fibrous tissue hyperplasia of the capsular wall with calcification and giant cell reaction.
A plain X-ray of the pelvis revealed calcification of a soft tissue mass in the right hip. No cortical hyperostosis, diaphysitis, or periosteal apposition was observed at the femur (Figure 2b) or ankle joints. Magnetic resonance imaging of the pelvis confirmed the findings of the plain radiography (Figure 2c).
An operation to remove the mass on the right hip of the proband was performed in our hospital. The tumor size was ~16×3×5 cm3 with multiple lobes and gray allantoic fluid (Figure 2d). The size of the cyst within the tumor was 1.5×2.5 cm2, and the cyst was encapsulated by a 0.5 cm thick, smooth wall and contained a lime-like material. The histopathological examination of the tumor mass revealed fibrous tissue hyperplasia within the cyst wall with calcification and giant cell reaction (Figure 2e).
Genetic analysis
Whole-blood samples were collected from the proband, his sister and family members (9 samples) after written informed consent was obtained. Genomic DNA was isolated from the leukocytes. A direct sequencing method was used to sequence the GALNT3 and FGF23 genes of the patients and their relatives (ABI 3700 automated fluorescent sequencer was used for bidirectional sequencing). DNA from another 100 unrelated normal subjects was also tested for these two genes. The potential for DNA sequence alterations and the effects of such alterations on the structure and function of the DNA were evaluated by the online programs MutationTaster9 (http:www.mutationtaster.org), PolyPhen-210 (http://genetics.bwh.harvard.edu/pph2) and SIFT11 (http://sift.jcvi.org).
Results
As shown in Figure 3, exon 2 of the GALNT3 gene in both the proband and his sister had 2 homozygous missense mutations: a 539G-A homozygous mutation (Figure 3c1), which resulted in the substitution of arginine 180 with histidine (R180H), and a 659T-A homozygous mutation (Figure 3c2), leading to the substitution of isoleucine 220 with asparagine (I220N). Their parents did not present any clinical characteristics of FTC, but both had the heterozygous mutations of GALNT3 in exon2 (R180H and I220N; Figure 3b1 and b2). The identified nucleotide changes were not found in other members of the family (Figure 3a1). As predicted by MutationTaster and PolyPhen-2, both of these mutations were “Disease causing”. The results derived from the SIFT analysis also showed that the mutation of I220N is “Damaging” and that R180H is “Tolerated”.
The FGF23 mutation was not found in the proband or other members of the family. No mutations at these loci were identified in an additional 100 unrelated normal subjects.
Discussion
This study documents the first Chinese family clinically diagnosed with HFTC and confirmed by genetic analysis, with the identification of two new mutations in GALNT3.
FTC is an autosomal recessive, genetic metabolic disorder. The most notable clinical manifestation of FTC is ectopic calcification, predominantly at the hip, elbow joints, shoulders, and knees. FTC rarely affects the skin, intervertebral discs, epidural sites, or perineum. There are two subtypes of FTC, hyperphosphatemic (HFTC) and normophosphatemic FTC. Clearly, the two cases presented here are not normophosphatemic FTC. In addition, our patients did not present bone lesions, such as hyperostosis; therefore, hyperostosis-hyperphosphatemia syndrome, which also results from mutations in the GALNT3 gene,1 could be excluded.
Generally, HFTC occurs before the age of 20. Serum phosphate levels in these patients are elevated, but their circulating calcium and PTH concentrations are normal. The serum levels of 1,25-dihydroxyvitamin D3 are normal or slightly higher than normal in these patients. The renal function is normal in these patients.12,13
The imaging features of FTC are typical. These tumor masses are often located in the musculus extensor lateralis. Computed tomography typically reveals lobulated calcific masses within the soft tissues with a separate encapsulated cystic component. T1-weighted images from the magnetic resonance imaging show a non-homogeneous low-signal region, whereas the T2-weighted image shows a high-signal region of nodules and a non-signal region in the diffuse low-signal region.14 The two patients we presented also had typical imaging features of FTC.
A differential diagnosis of FTC should be made because several other medical conditions may appear similar to FTC in the imaging tests,1 such as renal inadequacy, heterotopic calcification secondary to primary hyperparathyroidism, and vitamin D poisoning. Systemic diseases with clinical manifestations of ectopic calcification include mixed connective tissue disease, dermatomyositis, polymyositis, and systemic lupus erythematosus. Neoplastic diseases (including synovial sarcoma, osteosarcoma, and chondrosarcoma) and some degenerative disorders, such as calcified tendonitis, may also have similar radiological findings to those of FTC.14 If the patient has no history of the above-mentioned diseases and has a normal renal function, normal serum PTH, and 25-hydroxyvitamin D3 levels, heterotopic calcification secondary to the other diseases is unlikely.
The most common molecular pathogenesis of HFTC is related to loss-of-function mutations in GALNT3,1 which encodes UDP-GalNAc transferase 3 (GalNAc-T3) and mediates the glycosylation of FGF23, a potent phosphaturic protein. In the presence of a GALNT3 mutation, FGF23 proteins cannot be glycosylated and are vulnerable to degradation, resulting in hyperphosphatemia and FTC.5,15,16
GALNT3 mutations in FTC can occur at various loci within the gene, and there are no obvious hotspot mutations.17–24 In our study, both the proband and his sister carried 2 homozygous mutations (R180H and I220N) on exon 2 of the GALNT3 gene. It has been reported that most GALNT3 mutations can lead to early termination of protein translation or the defective translation of exons caused by an error in intron splicing.18 Mutational analysis using online software programs revealed that these two mutations are both located within the coding region and may cause alterations in the amino acid sequence, which might affect protein features. Thus, these mutations are predicted to be pathogenic. In addition, the mutation I220N within the glycosyl transferase domain may cause splice-site changes. In addition, the novel mutations we identified in exon 2 are adjacent to several previous reported mutations, further suggesting that this region of the GALNT3 may be susceptible to an increased mutation rate.
The clinical phenotype and severity of HFTC are not always related to the genotype. In our cohort, each patient harbored two homogenous mutations in exon 2 of the GALNT3 gene and demonstrated severe clinical features, whereas other HFTC patients carrying one homozygous mutation in GALNT3, with mild to severe calcification, need multiple operations.19 Indeed, the phenotypes of FTC are heterogeneous, with a wide variation in disease severity and involved tissues.25,26 For example, Ichikawa S. et al. 27described an FTC patient with a compound heterozygote for two mutations in exon 3 and exon 5 of the GALNT3 gene, resulting in coding changes within the catalytic domain of GalNAc-T3. However, this patient demonstrated only a small eyelid ectopic calcification. In another two symptomatic siblings with compound heterozygous GALNT3 mutations in exon 4 and 5, one exhibited features of FTC but the other demonstrated hyperostosis-hyperphosphatemia syndrome.26 Different GALNT3 mutations have been reported in the literature (Table 2). It is noteworthy that our two cases are the only ones carrying homozygous mutations. The parents of the patients in our study carried compound heterozygotes for the two mutations in exon 2, which were reported in 11 of 32 families with FTC or hyperostosis-hyperphosphatemia syndrome caused by GALNT3 mutations (Table 2), but they were reluctant to participate in additional examinations to exclude mild FTC or hyperostosis-hyperphosphatemia syndrome.
Our study has several limitations. Serum FGF23 levels were not measured, and a genetic analysis was not performed on the KL gene. In addition, a functional analysis of the GALNT3 gene was not conducted.
In summary, we identified two novel mutations (R180H and I220N) in exon 2 of the GALNT3 gene. This report provides the first clinical and genetic description of HFTC in a Chinese family. Further study is needed to explore the mechanism of such a mutation on FGF23 activity. Our findings may assist not only in the clinical diagnosis of HFTC but also in the interpretation of the genetic information used for prenatal diagnosis and genetic counseling.
References
Sprecher E . Familial tumoral calcinosis: from characterization of a rare phenotype to the pathogenesis of ectopic calcification. J Invest Dermatol 2010; 130: 652–660.
Smack D, Norton SA, Fitzpatrick JE . Proposal for a pathogenesis-based classification of tumoral calcinosis. Int J Dermatol 1996; 35: 265–271.
Topaz O, Shurman DL, Bergman R et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 2004; 36: 579–581.
Larsson T, Yu X, Davis SI et al. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab 2005; 90: 2424–2427.
Ichikawa S, Imel EA, Kreiter ML et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest 2007; 117: 2684–2691.
Farrow EG, Imel EA, White KE . Miscellaneous non-inflammatory musculoskeletal conditions. Hyperphosphatemic familial tumoral calcinosis (FGF23, GALNT3 and alphaKlotho). Best Pract Res Clin Rheumatol 2011; 25: 735–747.
Bergwitz C, Banerjee S, Abu-Zahra H et al. Defective O-glycosylation due to a novel homozygous S129P mutation is associated with lack of fibroblast growth factor 23 secretion and tumoral calcinosis. J Clin Endocrinol Metab 2009; 94: 4267–4274.
Yamaguchi T, Sugimoto T, Imai Y et al. Successful treatment of hyperphosphatemic tumoral calcinosis with long-term acetazolamide. Bone 1995; 16: 247S–250S.
Schwarz JM, Rodelsperger C, Schuelke M et al. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 2010; 7: 575–576.
Adzhubei IA, Schmidt S, Peshkin L et al. A method and server for predicting damaging missense mutations. Nat Methods 2010; 7: 248–249.
Kumar P, Henikoff S, Ng PC . Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 2009; 4: 1073–1081.
Campagnoli MF, Pucci A, Garelli E et al. Familial tumoral calcinosis and testicular microlithiasis associated with a new mutation of GALNT3 in a white family. J Clin Pathol 2006; 59: 440–442.
Polykandriotis EP, Beutel FK, Horch RE et al. A case of familial tumoral calcinosis in a neonate and review of the literature. Arch Orthop Trauma Surg 2004; 124: 563–567.
Olsen KM, Chew FS . Tumoral calcinosis: pearls, polemics, and alternative possibilities. Radiographics 2006; 26: 871–885.
Kato K, Jeanneau C, Tarp MA et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem 2006; 281: 18370–18377.
Barbieri AM, Filopanti M, Bua G et al. Two novel nonsense mutations in GALNT3 gene are responsible for familial tumoral calcinosis. J Hum Genet 2007; 52: 464–468.
Ichikawa S, Baujat G, Seyahi A et al. Clinical variability of familial tumoral calcinosis caused by novel GALNT3 mutations. Am J Med Genet A 2010; 152A: 896–903.
Garringer HJ, Mortazavi SM, Esteghamat F et al. Two novel GALNT3 mutations in familial tumoral calcinosis. Am J Med Genet A 2007; 143A: 2390–2396.
Yancovitch A, Hershkovitz D, Indelman M et al. Novel mutations in GALNT3 causing hyperphosphatemic familial tumoral calcinosis. J Bone Miner Metab 2011; 29: 621–625.
Frishberg Y, Ito N, Rinat C et al. Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res 2007; 22: 235–242.
Rafaelsen S, Johansson S, Raeder H et al. Long-term clinical outcome and phenotypic variability in hyperphosphatemic familial tumoral calcinosis and hyperphosphatemic hyperostosis syndrome caused by a novel GALNT3 mutation; case report and review of the literature. BMC Genet 2014; 15: 98.
Ramnitz MS, Gourh P, Goldbach-Mansky R et al. Phenotypic and Genotypic Characterization and Treatment of a Cohort With Familial Tumoral Calcinosis/Hyperostosis-Hyperphosphatemia Syndrome. J Bone Miner Res 2016; 31: 1845–1854.
Demellawy DE, Chang N, de Nanassy J et al. GALNT3 gene mutation-associated chronic recurrent multifocal osteomyelitis and familial hyperphosphatemic familial tumoral calcinosis. Scand J Rheumatol 2015; 44: 170–172.
Vieira AR, Lee M, Vairo F et al. Root anomalies and dentin dysplasia in autosomal recessive hyperphosphatemic familial tumoral calcinosis (HFTC). Oral Surg Oral Med Oral Pathol Oral Radiol 2015; 120: e235–e239.
Sprecher E . Tumoral calcinosis: new insights for the rheumatologist into a familial crystal deposition disease. Curr Rheumatol Rep 2007; 9: 237–242.
Joseph L, Hing SN, Presneau N et al. Familial tumoral calcinosis and hyperostosis-hyperphosphataemia syndrome are different manifestations of the same disease: novel missense mutations in GALNT3. Skeletal Radiol 2010; 39: 63–68.
Ichikawa S, Imel EA, Sorenson AH et al. Tumoral calcinosis presenting with eyelid calcifications due to novel missense mutations in the glycosyl transferase domain of the GALNT3 gene. J Clin Endocrinol Metab 2006; 91: 4472–4475.
Dumitrescu CE, Kelly MH, Khosravi A et al. A case of familial tumoral calcinosis/hyperostosis-hyperphosphatemia syndrome due to a compound heterozygous mutation in GALNT3 demonstrating new phenotypic features. Osteoporos Int 2009; 20: 1273–1278.
Acknowledgements
This work was funded by the Science and Technology Commission of Shanghai Municipality (No. 134119a2400). This work was also supported by National Rare Diseases Registry System of China (Nos. 2016YFC0901500 and 2016YFC0901503) and the Shanghai Municipal Heath Bureau Project (No. 20124235).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sun, L., Zhao, L., Du, L. et al. Identification of two novel mutations in the GALNT3 gene in a Chinese family with hyperphosphatemic familial tumoral calcinosis. Bone Res 4, 16038 (2016). https://doi.org/10.1038/boneres.2016.38
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/boneres.2016.38
This article is cited by
-
Relationship between rs7586085, GALNT3 and CCDC170 gene polymorphisms and the risk of osteoporosis among the Chinese Han population
Scientific Reports (2022)
-
Epidemiology of congenital disorders of glycosylation (CDG)—overview and perspectives
Journal of Rare Diseases (2022)
-
Hyperphosphatemic familial tumoral calcinosis caused by a novel variant in the GALNT3 gene
Journal of Endocrinological Investigation (2020)
-
Clinical and genetic analysis of idiopathic normophosphatemic tumoral calcinosis in 19 patients
Journal of Endocrinological Investigation (2020)
-
Clinical Utility Gene Card For: GALNT3 defective congenital disorder of glycosylation
European Journal of Human Genetics (2018)