Abstract
Background
Renal interstitial fibrosis (RIF) is a pathological change common to a variety of chronic renal diseases, ultimately progressing to end-stage renal failure. It is believed that epithelial cell phenotype inversion plays an important role in RIF, which is characterized by expression of the mesenchymal maker α-SMA, loss of the epithelial maker E-cadherin, and enhanced secretion of extracellular matrix. IL-17, a newly discovered pro-inflammatory cytokine, has recently been reported to play an important role in tissue fibrosis, involving pulmonary, liver, intestine and skin tissues. This study aimed to investigate whether IL-17A, a member of the IL-17 family, can induce epithelial cell phenotype inversion, and to explore the molecular mechanism of this phenotype inversion, in vitro.
Methods
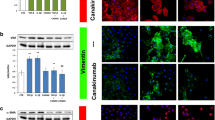
HK-2 cells were cultured and incubated with IL-17A. Cell proliferation was measured by CCK-8 assay, and the secretion of types I and III collagen was detected by ELISA in dose-dependent and time-dependent experiments. To find out whether IL-17A can induce epithelial cell phenotype inversion, HK-2 cells were stimulated with 80 ng/mL of IL-17A and 10 ng/mL of TGF-β1 as a positive control, for 72 h. To explore the potential signaling pathway, anti-TGF-β1 antibody was added before IL-17A treatment. At the same time, anti-TGF-β1 antibody alone was added to the medium as the negative control group. The expression of types I and III collagen, α-SMA and E-cadherin proteins, and mRNA was measured by real-time PCR, western blotting and immuno-histochemistry.
Results
IL-17A promoted the proliferation of HK-2 cells and secretion of types I and III collagen in a dose-dependent and time-dependent manner. Compared with the normal control, IL-17A could stimulate the expression of α-SMA, types I and III collagen, and suppressed the expression of E-cadherin in HK-2 cells. Incubation of IL-17A with TGF-β1 antibody decreased significantly the expression of α-SMA, but increased the expression of E-cadherin in HK-2 cells.
Conclusion
Our results suggest that IL-17A might promote the proliferation of HK-2 cells and secretion of extracellular matrix, and induce epithelial cell phenotype inversion via a TGF-β1-dependent pathway. Blocking the pro-inflammatory cytokine IL-17A might be a potential target for the treatment of fibrotic kidney disease.
Similar content being viewed by others
References
Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol 2000; 15: 290–301.
Razzaque MS, Taguchi T. Cellular and molecular events leading to renal tubulointerstitial fibrosis. Med Electron Microsc 2002; 35: 68–80.
Okada H, Kalluri R. Cellular and molecular pathways that lead to progression and regression of renal fibrogenesis. Curr Mol Med 2005; 5: 467–74.
Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol 2001; 159: 1465–75.
Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 2010; 21: 1819–34.
Cho ME, Smith DC, Branton MH, Penzak SR, Kopp JB. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2007; 2: 906–13.
Conway B, Hughes J. Cellular orchestrators of renal fibrosis. QJM 2012; 105: 611–5.
Boor P, Floege J. The renal (myo-)fibroblast: a heterogeneous group of cells. Nephrol Dial Transplant 2012; 27: 3027–36.
Badid C, Mounier N, Costa AM, Desmouliere A. Role of myofibroblasts during normal tissue repair and excessive scarring: interest of their assessment in nephropathies. Histol Histopathol 2000; 15: 269–80.
Ohtake Y, Tojo H, Seiki M. Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. J Cell Sci 2006; 119: 3822–32.
Lan HY. Tubular epithelial-myofibroblast transdifferentiation mechanisms in proximal tubule cells. Curr Opin Nephrol Hypertens 2003; 12: 25–9.
Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med 2004; 82: 175–81.
Strutz F, Muller GA. Renal fibrosis and the origin of the renal fibroblast. Nephrol Dial Transplant 2006; 21: 3368–70.
Slattery C, Campbell E, McMorrow T, Ryan MP. Cyclosporine Ainduced renal fibrosis: a role for epithelial-mesenchymal transition. Am J Pathol 2005; 167: 395–407.
Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelialmesenchymal transition: newinsights in signaling, development, and disease. J Cell Biol 2006; 172: 973–81.
Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005; 6: 1123–32.
Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005; 6: 1133–41.
Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol 2002; 71: 1–8.
Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin Immunol 2007; 19: 362–71.
Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity 2004; 21: 467–76.
Manotham K, Tanaka T, Matsumoto M, et al. Transdifferentiation of cultured tubular cells induced by hypoxia. Kidney Int 2004; 65: 871–80.
Fan JM, Ng YY, Hill PA, et al. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int 1999; 56: 1455–67.
Dong Z, Yang Y, Zhang T, et al. siRNA-Act1 inhibits the function of IL-17 on lung fibroblasts via the NF-kappaB pathway. Respiration 2013; 86: 332–40.
Du WJ, Zhen JH, Zeng ZQ, et al. Expression of interleukin-17 associated with disease progression and liver fibrosis with hepatitis B virus infection: IL-17 in HBV infection. Diagn Pathol 2013; 8: 40.
Meng F, Wang K, Aoyama T, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 2012; 143: 765–76, e1-3.
Biancheri P, Pender SL, Ammoscato F, et al. The role of interleukin 17 in Crohn’s disease-associated intestinal fibrosis. Fibrogenesis Tissue Repair 2013; 6: 13.
Okamoto Y, Hasegawa M, Matsushita T, et al. Potential roles of interleukin-17A in the development of skin fibrosis in mice. Arthritis Rheum 2012; 64: 3726–35.
Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006; 132: 191–206.
Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res 2007; 74: 196–206.
Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 2008; 214: 199–210.
Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulo-interstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 2003; 112: 1486–94.
Phanish MK, Wahab NA, Colville-Nash P, Hendry BM, Dockrell ME. The differential role of Smad2 and Smad3 in the regulation of pro-fibrotic TGFbeta1 responses in human proximal-tubule epithelial cells. Biochem J 2006; 393: 601–7.
Meng XM, Huang XR, Chung AC, et al. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol 2010; 21: 1477–87.
Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol 2010; 177: 1065–71.
Mi S, Li Z, Yang HZ, et al. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-beta1-dependent and-independent mechanisms. J Immunol 2011; 187: 3003–14.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors contributed equally to this article.
About this article
Cite this article
Liu, L., Li, Fg., Yang, M. et al. Effect of pro-inflammatory interleukin-17A on epithelial cell phenotype inversion in HK-2 cells in vitro . Eur Cytokine Netw 27, 27–33 (2016). https://doi.org/10.1684/ecn.2016.0373
Published:
Issue Date:
DOI: https://doi.org/10.1684/ecn.2016.0373