TISSUE ENGINEERING
Tissue engineering combines both biological sciences and engineering to develop treatments that restore, maintain or improve tissue function[1,3,4]. Though similar to regenerative medicine, an important distinction resides in the potential use of synthetic and semisynthetic materials in tissue engineering[4-6]. This separation can be better understood by considering the three major components of tissue engineering: Metabolically active cells[7], polymeric micro-carriers or scaffolds[8] and bioreactors to produce the tissue engineered construct for implantation[9].
The application of stem cells to tissue engineering applications has been a major recent advance in the field. Although a variety of stem cell types exist, including human embryonic cells and induced pluripotent stem cells, this review will focus on mesenchymal stem/stromal cells (MSCs). The potential for using MSCs for clinical purposes is an expanding area, for both their relative ease of acquisition and their versatility although many utilize their immunomodulatory and anti-inflammatory properties rather than generating new tissue[10-12]. Polymeric micro-carriers, hydrogels and scaffolds are essential components for supporting the reconstitution of damaged tissue. Seeding a scaffold with viable adult stem cells enables their differentiation into the cells desired when implanted into the body[13]. One key question in the tissue engineering field is the choice of polymer, particularly whether to use synthetic or biodegradable polymers. Bioreactors are generally defined as devices in which biological and/or biochemical processes for generating the tissue engineering construct are developed under closely monitored and tightly controlled environmental and operating conditions, i.e., Good Manufacturing Practice[14]. In modern tissue engineering, bioreactors are powerful tools to support and direct in vitro development of stem cell populations into functional tissues by simulating an appropriate biological, physical and mechanical environment. In essence, bioreactors are the means by which the desired tissue is generated in vitro and directed in its development for transplanting into the patient.
PELVIC ORGAN PROLAPSE
Pelvic organ prolapse (POP) is the herniation of pelvic organs into the vagina (Figure 1)[15,16]. Symptoms of POP include bowel and urinary incontinence, pain, voiding, bowel and sexual dysfunction, severely affecting the quality of life of affected women[17]. POP is a common condition, affecting approximately 25% of all women in the United States and Western countries, and is particularly prevalent in post-menopausal women. The main risk factor is vaginal birth and age. However, obesity is also a contributing factor, particularly in regard to POP recurrence[18]. Though not as well understood, a genetic predisposition to POP is a factor in some cases, particularly in genes regulating collagen and elastin synthesis in the pelvic floor and vaginal walls[19-21]. Given that the United States, Europe and Australia face increasing obesity rates and an aging population, the prevalence and severity of POP will only increase over the coming years. The economic and healthcare costs are considerable, approximating US$1 billion each year[22].
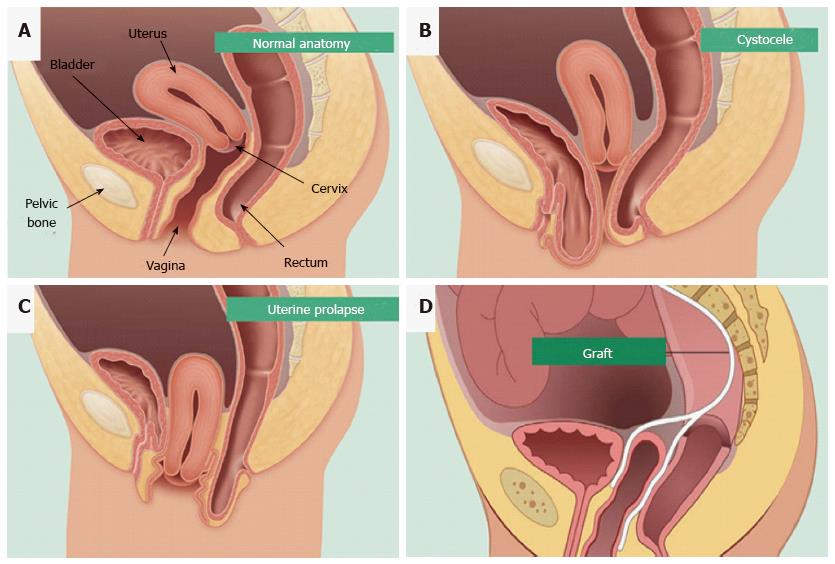
Figure 1 Pelvic organ prolapse mesh treatment.
Normal pelvic anatomy (A) and herniation of the bladder (B) and uterus into the vagina (C). Synthetic mesh augmentation of vaginal walls as a colporrhaphy treatment for pelvic organ prolapse (D). Hysterectomies are also used to treat uterine prolapse (reproduced with permission from BARD medical).
Surgical reconstruction for treatment of POP
Currently the standard treatment for POP is native tissue repair conducted transvaginally (colporrhaphy) or abdominally (sacral colpopexy). This surgical treatment has a high failure rate with 30% of patients requiring one or more further surgeries due to recurrence of POP[23]. Additionally, reconstructive procedures in older women have complication rates from 15.5% to 33%, with the majority related to urinary tract infections, febrile morbidity and blood loss requiring transfusion[24]. Indeed, the mortality from urogynecological surgery increases with each decade of life, with the most common complications occurring in women 80 years or older[25].
The first generation of augmented treatments for POP involved the implantation of polypropylene mesh into the vaginal walls to alleviate the herniation and support the pelvic organs (Figure 1D)[26]. Mesh has been available since the 1950s for the repair of abdominal hernias[26]. Though successful for many women, up to 30% will require subsequent surgery while also enduring other complications such as fibrosis, mesh erosion into the vagina, bladder or bowel, chronic inflammation and mesh shrinkage[24,26,27]. This resulted in worldwide recalls of many of the leading brands of meshes for vaginal surgery, leaving women with fewer options for treatment once again.
CANDIDATE CELLS FOR TISSUE ENGINEERING APPLICATIONS FOR POP
Skeletal muscle derived stem cells
Skeletal muscle has been identified previously as a potential source of progenitor stem cells capable of differentiating into myogenic and osteogenic cell lineages in rat models[28-33]. The use of skeletal muscle stem cells to deliver gene therapy is being explored for treating muscular dystrophy and stress urinary incontinence, another pelvic floor disorder involving the urethra[28]. In addition, they are being used to regenerate both skeletal and cardiac muscle, bone and cartilage. As a potential source of cells for treating POP, muscle-derived stem cells (MDSC) are particularly attractive as they can now be isolated from human skeletal muscles and differentiated into skeletal myotubes, in vitro and in vivo[33]. In rat models MDSC have been used to treat fibrosis. The ability of MDSC to promote vaginal epithelial regeneration and vaginal wall repair in a rat model makes them candidates for treating POP[34]. However to avoid the risk of immune rejection from allogeneic sources, MDSC are better derived from the patient’s own muscle tissue. Such an autologous procedure is expensive and invasive, causing significant pain and morbidity for the patient. An alternative source of cells for POP treatment could prove more beneficial and practical for the patient.
Fibroblasts and myofibroblasts
As major producers of collagen and an essential cell for the formation of connective tissue, fibroblasts have also been suggested as an alternative cell source for POP treatment[35]. Vaginal myofibroblasts from nulliparous women have higher contractile strength compared to those from parous women, suggesting that vaginal delivery and overstretching of the vaginal wall affects myofibroblast function[36]. However, the use of autologous vaginal fibroblasts from patients for treating their pelvic floor disorders raises concerns about the quality of cells utilised. Other studies have observed that vaginal fibroblasts derived from prolapsed tissues have impaired function, such as delayed fibroblast-mediated collagen contraction and lower production of collagen synthesising enzymes[21]. This could be avoided if women have a vaginal biopsy to collect and cryopreserve fibroblasts before childbirth in order to obtain better quality cells, however long-term planning and storage facilities are not available to most women. The invasive method of acquiring human vaginal fibroblasts and subsequent morbidity is unfortunately an obstacle in their use as the main source of cells for a tissue engineering-based approach to treating POP.
Buccal mucosal fibroblasts (BMF), however offer a readily available and plentiful source of cells and could prove an alternative to human vaginal fibroblasts. BMF are harvested from the inside of the cheek lining and express the typical MSC/fibroblast surface markers but do not function as MSC[37]. They produce important components of the extracellular matrix, collagen I and elastin, both of which are required for strengthening the vaginal walls to alleviate and prevent herniation[35,38]. The interaction of BMF with various biodegradable scaffolds has been examined in vitro for potential treatment of PFDs including POP[38]. Although BMF offer a potential candidate for the treatment of POP, they currently remain untested for this purpose in animal models and their ultimate suitability remains unknown.
MSCs
MSC have been extensively used as cell-based therapies predominantly for their anti-inflammatory and immunomodulatory non-stem cell properties[39,40]. However they also have potential for tissue engineering purposes for regenerating new tissues or promoting the activity of endogenous stem cells[10,13,41]. MSC populations have the capacity for self-renewal, high proliferative potential and differentiate into a variety of mesodermal and other lineages[42]. Recent advances in cellular identification using more specific markers has shown that MSC can be extracted from most tissues including bone marrow, umbilical cord, placenta, adipose tissue and endometrium, although not all of these sources have demonstrated clonogenicity for their MSC populations[43-47]. Typically, MSC actively respond to stress or injury in a similar manner to the way cells of the innate immune system respond to pathogen exposure. When supplied systemically, exogenous MSCs home to sites of injury in response to inflammation[48]. Here MSCs operate in a paracrine manner secreting large amounts of diverse proteins, growth factors, cytokines and chemokines that promote a variety of effects including neo-angiogenesis, tissue regeneration and remodelling, immune cell activation, suppression of inflammation and cellular recruitment[13,41,49-51].
The potential of MSC as a cell-based therapy has recently been explored in numerous clinical applications. The ability to direct bone marrow MSC differentiation into other cell types and lineages has shown that these cells maintain a phenotype lacking tissue-specific characteristics until exposed to signals in damaged tissues[52]. MSC obtained from dental pulp have been used to repair related tissues such as periodontal ligament, dental papilla and dental follicle[53]. The ability of adipose tissue and bone marrow MSC to act as precursor cells has also been exploited by directing their differentiation toward the chondrogenic lineage in order to produce cartilage-synthesising chondrocytes[54]. Although MSC show promise as cell-based therapies, more understanding of their mechanism of action and utilising their potential is needed. Early use of MSC has not always met expectations, often producing inconsistent results[55]. This may be due to lesser refined methods of isolating and cultivating MSC resulting in the administration of fibroblasts and myofibroblasts rather than undifferentiated MSC[56]. Until recently, production of significant numbers of MSCs posed a challenge, as the regenerative potential of MSC declined during culture expansion[57,58], which is required due to the small numbers of perivascular MSC present within tissues[59]. For tissue engineering applications and tissue repair following ischaemia (e.g., cardiac muscle), local rather than systemic delivery is desirable and will likely result in greater local concentration of MSC at the desired tissue site, even when the mechanism of action is paracrine[60]. A further consideration is allogeneic vs autologous. Seeding MSC onto scaffolds, such as polyamide/gelatin (PA + G) for POP or poly-lactic-co-glycolic acid nano-fibers appears to produce better outcomes in preclinical studies[57,61]. MSCs are a versatile and promising stem/stromal cell which can be used for a variety of regenerative medicine applications. Additionally, MSC have greater capacity to regenerate tissues from which they are derived[39]. With this in mind, MSC obtained from the lining of the uterus could be useful in the development of treatments for other regions of the female reproductive tract, e.g., vaginal wall tissue in cases of POP.
ENDOMETRIUM AS A NOVEL SOURCE OF MSC
Regenerative potential of endometrium
The endometrial lining of the uterus serves as the site of embryo implantation, placentation and the development of the embryo and foetus during pregnancy[62]. The upper functional layer of the human endometrium undergoes extensive growth, differentiation and shedding each menstrual cycle under the influence of sex steroid hormone fluctuations[63]. Following menstruation, the remaining basal layer regenerates the new functional layer, which undergoes rapid cellular proliferation followed by differentiation (Figure 2). If an embryo does not implant, the terminally differentiated epithelium and stroma is shed during menstruation[64]. Much like the continuously renewing small intestinal mucosa, the endometrial mucosa undergoes many cycles of regeneration during a woman’s lifetime, indicative of its highly dynamic and regenerative capacity.
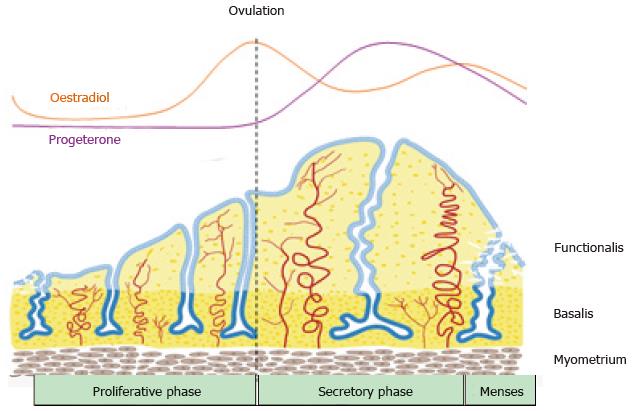
Figure 2 Schematic of changes in the human endometrium during the menstrual cycle, illustrating the growth, differentiation and shedding of the functionalis layer.
The functionalis layer regenerates 4-10 mm during the proliferative phase (10 d) as cells proliferate in response to rising circulating estrogen levels. During the secretory phase, progesterone induces differentiation of the epithelium and stroma to generate an endometrium receptive to implantation of an embryo. This entire process occurs over 400 times during a woman’s reproductive life indicating the regenerative potential of human endometrium (reproduced from ref.[63] with permission).
Endometrial MSC
The existence of stem/progenitor cells within the endometrium and their role as progenitor cells for regenerating endometrial tissue has only recently been reported. Endometrial MSC (eMSC) are clonogenic, multipotent, differentiating into four mesodermal lineages: Osteoblasts, chondrocytes, smooth muscle cells and adipocytes in vitro (Figure 3) and expressing the typical pattern of MSC surface markers[44,65,66]. Endometrial side population (SP cells) also demonstrate MSC properties[67,68]. Serial clonal culture shows that clonogenic eMSC undergo self-renewal in vitro and have high proliferative potential[44]. The population of clonogenic eMSC within human endometrium is small approximating 1.3%, necessitating the identification of specific surface markers to allow their prospective isolation and enrichment from endometrial biopsies[69,70].
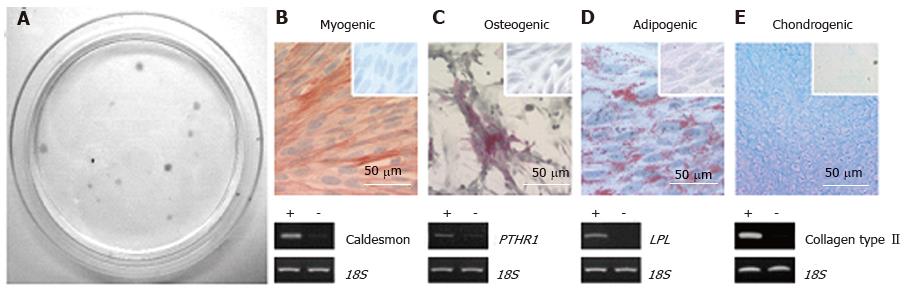
Figure 3 Endometrial mesenchymal stem cells.
Clonogenic (A); and differentiate into 4 mesodermal lineages from a single clonogenic cell (B-E); myocytes (B); osteocytes (C); adipocytes (D); chondrocytes (reproduced from ref. [44] with permission) (E). PTHR1: Parathyroid hormone 1 receptor; LPL: Lipoprotein lipase.
Prospective isolation of eMSC
In order to exploit the regenerative ability of eMSC, they must first be isolated from the heterogeneous population of cells obtained from dissociated endometrial tissue. Ideally this requires the identification of unique surface markers on eMSC that will identify their in vivo niche and separate them from undesired stromal fibroblasts and other cells. Indeed several sets of specific surface markers have been identified on eMSC[70-73]. Almost all clonogenic human endometrial stromal cells with MSC properties are found in the CD140b+CD146+ population, comprising 1.5% of the stromal fraction[70]. These markers revealed a perivascular niche for eMSC adjacent to endothelial cells suggesting they are pericytes (Figure 4). The transcriptome of the co-expressing CD140b+CD146+ cells indicates they are distinct from CD140b-CD146+ endothelial cells, but more similar to endometrial CD140b+CD146- stromal fibroblasts[73]. To obtain these co-expressing cells, a flow cytometry sorter must be used, which limits the utility of this marker set, given the damaging effects of automated cell sorting on cell viability[70]. To overcome this problem a single perivascular marker was sought for isolating eMSC. The W5C5 antibody identified a population of perivascular endometrial stromal cells with typical MSC properties that also reconstituted stromal tissue in vivo when transplanted beneath the kidney capsule[72]. The W5C5+ cells comprised 4.4% of endometrial stromal cells. The epitope recognised by the W5C5 antibody is the Sushi Domain-containing 2 (SUSD2) adhesion molecule[74]. A single marker enables magnetic bead sorting, a gentler protocol than using a cell sorter as evidenced by increased clonogencity of SUSD2+ cells compared to CD140b+CD146+ cells[72]. TNAP (tissue non-specific alkaline phosphatase) is another single marker that identifies eMSC, but has less utility as the epitope is also expressed by endometrial epithelial cells[75]. Another perivascular marker (AOC3) identified by RNA sequencing SUSD2+ and SUSD2- cells may have utility for isolating eMSC[76], but the common bone marrow MSC marker Stro-1 does not enrich for endometrial stromal cells with MSC properties[69]. All these markers revealed that the perivascular eMSC were found in both the functionalis and basalis layers of human endometrium, indicating that eMSC will be found in menstrual blood and can be isolated from biopsies and curettage as well as hysterectomies[56,77].
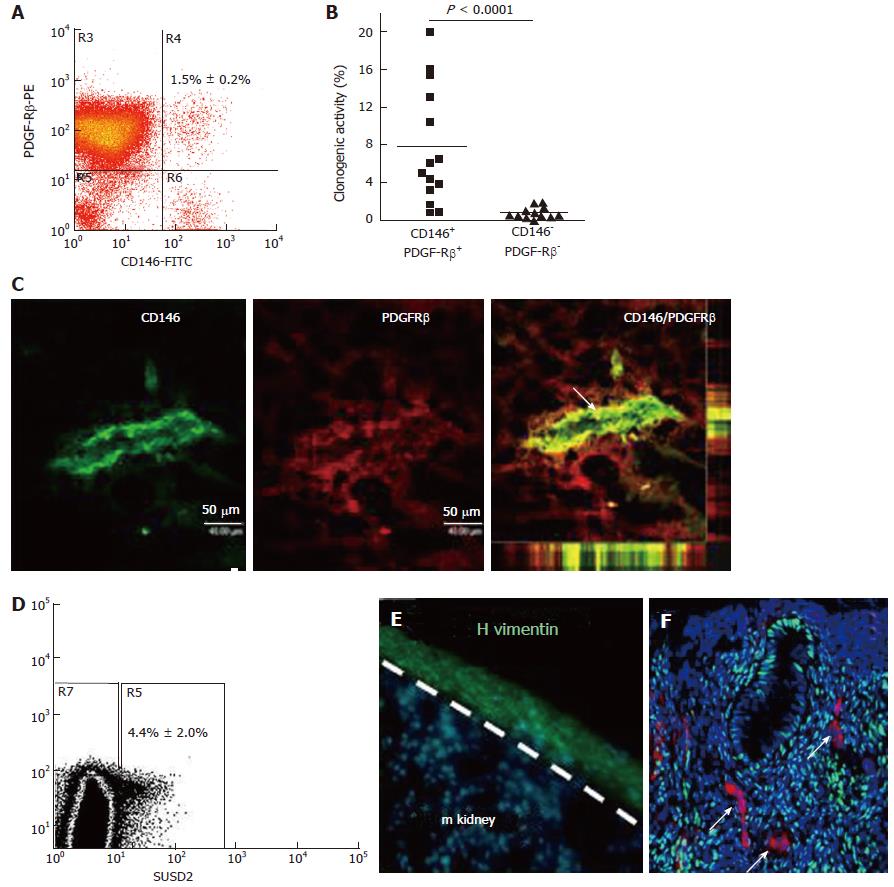
Figure 4 Specific enriching for endometrial mesenchymal stem cells.
Flow cytometry plot of CD146+PDGFRB+ fraction (A) which contains most of the clonogenic stromal cells (B) and reveals their pericyte identity in vivo (C); SUSD2+ cells in endometrial cell suspensions (D) which E reconstitute human vimentin+ stromal tissue when transplanted under the kidney capsule of NSG mice, and F have a perivascular location in human endometrium. SUSD2+ cells (red) do not express estrogen receptor-α (green), but endometrial stromal cells do (DNA blue). The white arrow indicates perivascular SUSD2+ cells (reproduced ref. [70,72,78] with permission).
EMSC can also be obtained from post-menopausal women following short term (8 wk) estrogen replacement which regenerates their atrophic endometrial tissue[78]. Collection of menstrual blood or an endometrial biopsy are convenient sources not requiring anaesthesia, with the latter available as a simple office based procedure. Such tissue sources are ideal for cell-based therapies (Figure 5). Despite their great promise, eMSC and menstrual blood MSC have yet to be significantly explored as therapeutic agents for stem cells therapies. There are certain endometrial disorders where caution maybe required eg endometriosis. However this disorder affects young infertile women who will not have the opportunity to develop POP. Indeed, it will be important to ensure no underlying uterine or other pathology (e.g., malignant tumour) in identifying suitable patients for cell harvesting to treat their POP. For example, should a woman have uterine cancer, it would not be possible to use her eMSC for cell-based therapies. Similarly, it would also be contraindicated to use another source of autologous MSC in case tumour cells have spread to organs such as bone. These important issues should be considered in developing the potential of eMSC as cell-based therapies.
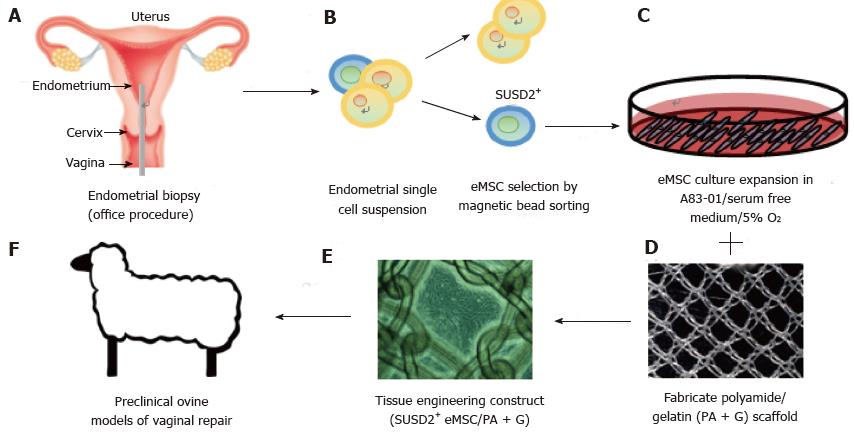
Figure 5 Isolation and application of e-mesenchymal stem cells in pelvic organ prolapse vaginal repair.
(A) simple office based endometrial biopsies can be used to obtain patients’ tissues, which are dissociated, then (B) eMSC selected using SUSD2 magnetic bead sorting, followed by (C) culture expansion in A83-01/serum free medium in 5% O2 to generate large numbers of undifferentiated SUSD2+ eMSC (90%-95%) for (D) seeding onto fabricated scaffolds which will create an (E) eMSC/PA-G tissue engineering construct for implantation into (F) a large animal preclinical model to assess their efficacy in vaginal repair of parous ewes with evidence of POP (reproduced with permission from ref.[57,103] with permission). POP: Pelvic organ prolapse; MSC: Mesenchymal stem cells.
Large animal models are usually required to provide data for regulatory bodies prior to translating potential cell-based therapies into the clinic. If autologous applications are being evaluated, it becomes necessary to derive MSC from species such as ovine, porcine, canine, equine and non-human primates[79,80]. Often antibodies used as biomarkers to derive MSC from human or mouse do not cross react with these species. For example, neither CD140b, CD146 nor SUSD2 cross react with ovine endometrial tissue[81]. However, the bone marrow MSC surface marker CD271[82] cross reacts with ovine endometrial stromal cells enriching for eMSC demonstrating clonogenicity, in vitro self-renewal and the ability to differentiate into adipogenic, myogenic, osteogenic and chondrogenic lineages[81]. The CD271+ ovine eMSC were identified in a perivascular niche around arterioles and venules in vivo, but unlike human eMSC which have a pericyte location, ovine CD271+ stromal cells occupied the adventitia in the periphery of these vessels (Figure 6). In human bone marrow and adipose tissue, vascular adventitial cells show similar MSC properties as those located in the pericyte position[83].
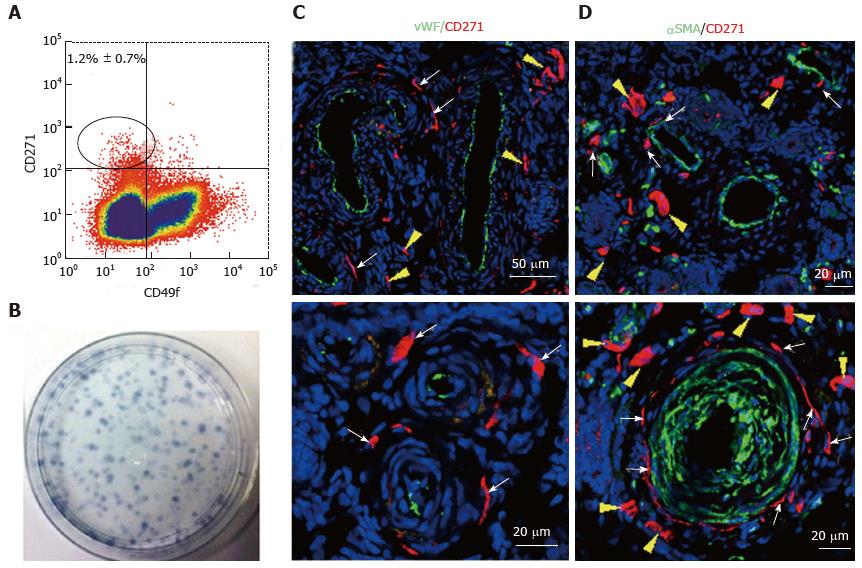
Figure 6 Specific markers for ovine e-mesenchymal stem cells.
Flow cytometry plot of ovine endometrial cells immunolabelled with CD271 and CD49f antibodies (A). The CD271+CD49f- population enriches; Clonogenic stromal cells (B); Immunofluorescence images of ovine endometrium stained with CD271 (red) and vascular markers reveals their in vivo perivascular location in the adventitia of veins and arteries (C, D); vWF an endothelial marker (green), showing CD271+ cells are perivascular but not pericytes (C); αSMA, a perivascular marker (green) showing CD271+ cells located adjacent to αSMA+ cells in the adventitia of vessels rather than expressing αSMA themselves (D). White arrows: perivascular CD271+ cells; yellow arrows: CD271+ cells not associated with vessels (reproduced from ref.[81] with permission). vWF: Von Willebrand factor; αSMA: Alpha smooth muscle actin.
eMSC phenotype and gene profile
Cell fate decisions made by somatic stem cells to self-renew or undergo differentiation depends upon the cellular microenvironment or niche from signals emanating from cells and extracellular matrix that comprise this niche[84]. In this context, understanding both the extrinsic and intrinsic gene regulation pathways operating in undifferentiated eMSC and their more differentiated progeny could shed light on their function in endometrial regeneration. Gene expression profiling comparing purified endometrial cell populations of CD140b+CD146+ eMSC, CD140b+CD146- stromal fibroblasts and CD140b-CD146+ endothelial cells showed that eMSC differentially expressed 762 and 1518 genes, respectively[73]. The eMSC gene expression profile was typical of stem cells, showing upregulation of self-renewal genes of the TGFβ, FGF2, WNT, IGF and Hedgehog signalling pathways in comparison with the endometrial stromal fibroblasts and endothelial cells. The expression of SUSD2 was also elevated in the double positive eMSC population. G-protein coupled receptor- and cAMP-mediated signalling were also upregulated in the CD140b+CD146+ population, similar to genes involved in maintaining the undifferentiated state of bone marrow MSC. The CD140b+CD146+ population also showed upregulation of immunomodulatory and immunosuppressive genes[73]. eMSC displayed increased expression of Cyclin D1 (CCND1) which ensures their progression through the G1 phase of the cell cycle[73]. Gene profiling has confirmed human eMSC as pericytes, while RNA sequencing of cultured endometrial SUSD2+ and SUSD2- cells revealed 134 differentially expressed genes, with many of those in the SUSD2+ population characteristic of perivascular cells[76]. The in vivo differentiation pathway for eMSC is to decidualised perivascular cells and decidual cells of the endometrial stroma, a process mediated by the post-ovulation sex steroid hormone, progesterone, via production of cAMP. The perivascular location of eMSC in the spiral arterioles renders them well situated to participate in the regeneration of the uterine lining and formation of the placenta during embryo implantation and subsequent pregnancy[76].
Tissue engineering for POP repair
Given the problem associated with mesh implantation for POP repair, and the need for physical support, a tissue engineering approach may provide a more durable treatment. The ideal treatment for POP would be an implantable autograft that alleviated herniation and regenerated the damaged tissue within the vaginal wall.
In vitro studies
For a cell based treatment to be practical, methods for procuring and expanding the necessary cells need to be developed. Culturing and expanding eMSC in vitro has been optimised in serum-free conditions, showing that fibronectin is the optimal substrate for attachment[85]. Additionally, hypoxic conditions of 5% O2 increased the proliferation rate and yield of eMSC, whilst maintaining multipotency and their expression of CD140b, CD146 and SUSD2. Culturing eMSC on a polyamide/gelatin composite scaffold with exogenous TGFβ1 and PDGF-BB induced their differentiation into smooth muscle cells expressing SM22α and SM-myosin heavy chain[86]. Incubation with connective tissue growth factor induced the eMSC to differentiate into collagen-producing fibroblasts. The differentiated smooth muscle cells and fibroblasts no longer expressed the eMSC marker SUSD2, confirming their differentiation into these desired cell types for POP repair[86]. Although these in vitro studies show promise, it is also essential to confirm smooth muscle and fibroblast differentiation in vivo to gain mechanistic understanding prior to transferring this technology into clinical applications.
Methodology has now been developed for culture expansion of eMSC in serum free medium containing A83-01, a TGFβ1 receptor inhibitor, that maintains eMSC stemness and SUSD2 phenotype[87]. TGFβ1-mediated apoptosis and senescence is prevented and proliferation promoted in A83-01-treated eMSC cultures maintaining the percentage of SUSD2+ cells to more than 90% for all samples. This effect of A83-01 is mediated via Smad2/3 phosphorylation. A83-01 treated eMSC are more clonogenic than untreated control cells and retain their MSC properties[87]. A major advantage of this culture method is that a reproducible percentage of SUSD2+ eMSC is achievable for all patient samples, an important consideration for scale out culture expansion of autologous cells.
In vivo studies
As outlined earlier there are substantial problems with current mesh augmentation of POP surgery. The use of autografts increases morbidity at the donor tissue site, biological materials often fail due to their rapid and unpredictable degradation[16], and the synthetic PP mesh currently used is biomechanically too stiff and often erodes into adjacent organs[56]. A better solution may be to combine the advantages of both the synthetic and biological approaches. This could utilise a synthetic mesh as a scaffold to not only support the prolapsed tissue but also provide a vehicle upon which to seed eMSC for delivery to sites of vaginal damage[26,88]. The eMSC could serve by modulating the inflammatory and immune responses and perhaps more importantly incorporating into the vaginal wall to regenerate the lost or damaged tissue or promoting endogenous stem cell populations to initiate repair which mesh alone cannot do.
Small animal rodent models
Recent efforts to test this possibility show potential utility. A non-degradable, polyamide (PA) mesh with biomechanical properties more closely matching vaginal tissue was coated with gelatin[88] to provide a substrate for seeding with SUSD2+ eMSC. This tissue engineering construct was then implanted into a fascial defect on the dorsum of immunocompromised rats and assessed following necropsy at several time points over 90 d[57]. In the explanted eMSC/PA + G tissue complexes, greater neovascularisation was observed early on at 7 d compared with PA + G controls. Initially there was a greater influx of M1 inflammatory macrophages around the eMSC-seeded mesh. At 60 d these macrophages had changed to a M2 wound healing phenotype and by 90 d there were fewer CD68+ macrophages around the cell-seeded PA + G filaments in comparison to PA + G alone, indicating a milder chronic inflammatory response in the long term. Importantly in these studies the cellular response at the mesh interface was assessed quantitatively in 50 μm increments around individual filaments using image analysis rather than subjective scoring[57,88]. Similar quantities of new collagen were generated around the PA + G mesh filaments, irrespective of the inclusion of eMSC, which was derived from rat fibroblasts rather than derivatives of the implanted human eMSC. However, this new collagen around the eMSC/PA + G mesh filaments showed physiological crimping by scanning electron microscopy, while more scar-like collagen was deposited around the PA + G mesh without eMSC[89]. This deposition of physiological collagen likely contributed to the improved biomechanical properties of the mesh/tissue complexes harvested at 90 d, where a longer toe region and lower stiffness was observed in the stress strain curves of the cell-seeded PA + G mesh compared with PA + G alone (Figure 7)[57]. The improved tissue organisation around the mesh filaments shown by histological staining suggests that eMSC promoted tissue regeneration and improved the biocompatibility of the synthetic PA + G mesh[57,89]. In this xenogeneic model, the eMSC survived a maximum of 14 d indicating that they exerted a paracrine effect in promoting vascularisation and reducing fibrosis similar to MSC effects on many other tissues[13]. However the percentage of SUSD2+ cells in the single sample of passage 6 cells used for the entire study was only 10%. It will be of interest to determine whether more than a paracrine effect will be observed if > 90% of the cells are SUSD2+, now a possibility by culturing them in A83-01-containing medium[87].
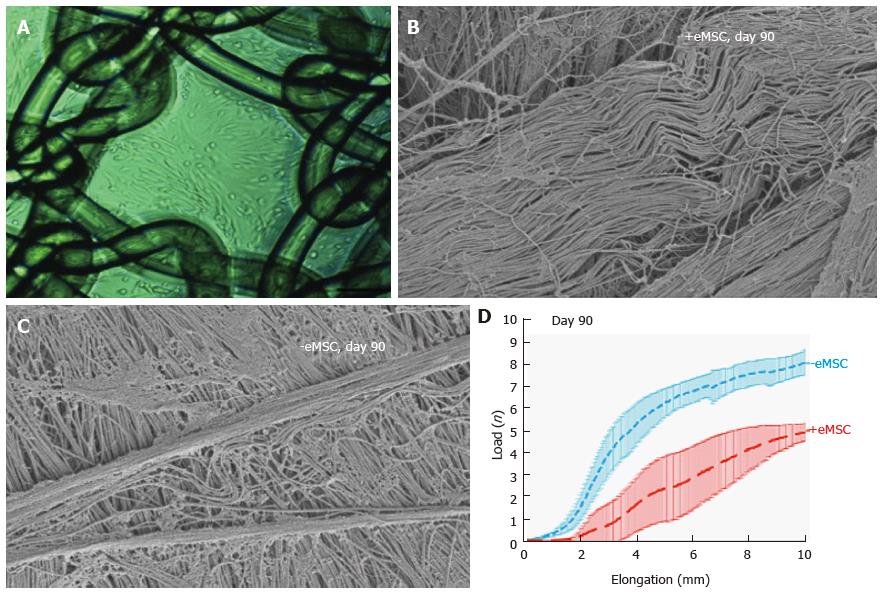
Figure 7 Human e-mesenchymal stem cells improves the biocompatibility of polyamide/gelatin (PA + G) mesh in a fascial wound defect in nude rats.
PA + G mesh seeded with 100000 eMSC/cm2 and cultured for 48 h, prior to implantation (A); Physiological crimped collagen deposited around eMSC+/PA + G mesh (B); Scar-like collagen in PA + G mesh alone as observed by SEM (C); Load-elongation curves of explanted meshes with (red) and without (blue) eMSC showing less stiffness (slope) and longer toe region for mesh seeded with eMSC, indicating improved biomechanical properties (reproduced from ref.[57,86,89] with permission) (D). MSC: Mesenchymal stem cells; PA : Polyamide; G: Gelatin.
Despite the significant biological differences between human females and rodents, mouse models have proven invaluable for the investigation of the underlying biochemical mechanisms involved in the development of POP. The use of genetically modified mice has allowed exploration of the genetic underpinnings of POP, such as lysyl oxidase like-1 (LOXL1), an enzyme involved in elastin biosynthesis within vaginal tissue walls, and fibulin 5 (FBLN5) which regulates expression of collagen and elastin. Depletion of either LOXL1 or FBLN5 has been associated with POP[20,90]. The LOXL1 deficient mouse creates a POP-like condition where the mice develop an obvious bulge in the perineal region. It would be of interest to determine if an injectable MSC-based cell therapy alleviates the prolapse symptoms of LOXL-1 deficient mice. While extremely useful for investigating genetic contribution, transgenic mice are limited in their utility as models for exploring tissue engineering based treatments for POP due to the small size of their vagina.
Large animal preclinical models
Of the large animal models available for assessing cell-based therapies for POP, the domestic sheep is the most promising candidate due to their ready availability and physiological similarity to the human female pelvis in size and structure. Ewes also have a similar oestrus cycle of 17 d, a long labour and deliver a foetus with a large head to body ratio that is closer to humans than rodents[91,92]. Like humans, ewes undergo spontaneous POP with similar frequency and predisposing factors, such as parity, age and breeds with a large rump[91,93]. Although the ovine species are quadrupeds with a horizontal rather than vertical pelvic floor subject to differing forces, the overall arrangement of the pelvic organs and the similar vaginal dimensions to women make them a useful model for assessing new mesh and tissue engineering constructs[56]. Additionally, the ovine vagina has a similar histological structure, biochemical and biomechanical properties to that of women. Finally, the most common form of prolapse in sheep involves the bladder (cystocele) as it is for women Sheep have already been vaginally implanted with various POP mesh materials for evaluation of their efficacy and adverse effects in female pelvic reconstructive surgery[16,92,94,95]. The biochemical and biomechanical properties of ovine vaginal tissue has already been examined by quantitative histological imaging, biochemical collagen/GAG/elastin assays and biomechanical analyses, providing a platform for the evaluation of next generation eMSC-seeded mesh in the ovine vaginal repair model[96,97]. It is now possible to evaluate autologous eMSC since methods have been developed for obtaining MSC from the ovine bone marrow[79] and endometrium (Figure 5)[81].
Additional large animal models for assessing cell-based therapies for POP surgery include cows, pigs and non-human primates. Cows develop prolapse with similar predisposition and frequency to humans and sheep[98], however their purchase, handling and agistment costs make them a less practical model. Pigs are a common preclinical model for various clinical conditions but their foetuses do not have the large head-to body-ratio responsible for inducing spontaneous POP in the ovine model, reducing their utility[99]. Non-human primates such as Rhesus macaques and squirrel monkeys offer useful animal models due to a similar pelvic anatomy to humans and their more upright posture[100,101]. Furthermore, the Rhesus species develop spontaneous POP. Non-human primates have been used for assessing new POP meshes and for investigating the mechanism of action of their deleterious effects[27,102]. However ethical limitations, prohibitive cost of handling and necessary specialist expertise limit their availability for many investigators. Despite these limitations, assessment of tissue engineering constructs in the macaque model, particularly in retired breeders with evidence of POP, might provide the ultimate model of postmenopausal women with POP in which to assess a cell-based therapy. However it would be necessary to develop methods for obtaining MSC populations from the macaque species, for both autologous and allogeneic use.