- Original Paper
- Open access
- Published:
Using water-soluble C60 fullerenes in anticancer therapy
Cancer Nanotechnology volume 2, pages 105–110 (2011)
Abstract
Growth experiments of transplanted malignant tumors in the presence of water-soluble C60 fullerenes were performed on groups of mice. It was found that C60 fullerenes efficiently inhibit the growth of transplanted malignant tumors. This behavior can be explained through their high antioxidant activity and the blocking of the specific cell receptors (for example, endothelial growth factor). The findings demonstrate the possibility of using C60 fullerenes in anticancer therapy.
1 Introduction
Malignant tumors are a major reason of the enhancement of the disability and mortality rates. According to the World Health Organization data, there were an estimated 12.4 million new cases of cancer worldwide in 2008 and 7.6 million deaths caused by the disease (Boyle and Levin 2008). The available methods at the present for the treatment of tumors are not sufficiently effective. The surgical removal of tumors is not always possible since only a small percentage of onco-patients could be operated and that the risk of postoperative complications is high. Chemotherapy is a palliative method of treatment that promotes short-term relief but does not lead to complete recovery. In addition, it is toxic and causes serious violations in the work of many organs, including liver and heart. In this regard, there is a need to develop alternative methods of tumor treatment and to find new substances, which act locally in tumor, causing its destruction. One of the mechanisms of such destruction is the simulation of tumor cells death by necrosis or apoptosis with preserving of normal cell viability. Biologically active non-toxic C60 fullerenes (Andrievsky et al. 2005; Prylutska et al. 2007; Johnston et al. 2010), which are able to penetrate through the membrane of cells (Foley et al. 2002) and have strong antioxidant and antiviral properties (Prylutska et al. 2008; Cataldo and Da Ros 2008), could be used for preventing the growth of malignant neoplasms. In fact, some fullerene derivatives have shown promising anticancer or antitumor activity (Freitas 2003). Namely, Murugesan et al. (2007) have demonstrated the substantial anti-angiogenic activity of carbon materials such as graphite, multi-walled carbon nanotubes, and C60 fullerenes against either basic fibroblast growth factor- or vascular endothelial growth factor-induced angiogenesis in the chick chorioallantoic membrane model. It is known that an imbalance in the levels of these factors causes many serious diseases including malignant cell growth (Carmeliet 2005). Furthermore, Meng et al. (2010) have reported that fullerene derivatives are capable to regulate in low levels simultaneously more than ten angiogenic factors in the mRNA level that is further confirmed at the protein level. This result indicates that fullerene-containing materials serve as a potent antiangiogenesis inhibitor that can simultaneously target multiple angiogenic factors. In addition, Yin et al. (2008, 2009) found that metallofullerene nanoparticles penetrate plasma membrane of tumor cells, effectively inhibit their proliferation, and decrease the activities of those enzymes which are responsible for catalyzing the production of reactive oxygen species in vivo.
Considering the importance of using fullerenes for the cancer treatment, the purpose of this work was mainly focused on investigating the influence of pristine (unmodified) water-soluble C60 fullerenes on the rate of growth of transplanted malignant tumors.
2 Materials and methods
The C60 fullerene aqueous solutions (C60FAS) used for the experiments were prepared as follows (Scharff et al. 2004): a saturated solution of C60 fullerenes (purity 99.5%) in toluene was mixed with the same amount of distilled water and the resulted two-phase system was ultrasonicated until the completion of the toluene evaporation. Afterwards, the yellow-colored water phase was filtered for removing the C60 fullerenes which were not dissolved. C60FAS samples with concentrations of C60 fullerenes in water in the range from 0.1 to 1.0 mg/ml were prepared. The C60FAS samples were found to be stable for about 18 months at 4°C.
Theoretical calculations (Prilutsky et al. 1999; Bulavin et al. 2000) showed that C60FAS contains both single C60 molecules and their clusters (with sizes of about 0.7–4 nm depending of C60 fullerene concentration) in the hydrated state. Moreover, C60 fullerenes structure the water, absorbed by DNA molecules (Turov et al. 2010), and thus they can affect the DNA functioning in the biosystem.
The state of C60 fullerenes in water was examined by using STM technique (NT-MDT, Russia). The samples were deposited on Au(111) surface by precipitation from aqueous solution droplet.
It is important to note that used C60FAS does not show a cytotoxic effect with respect to both normal and transformed cells at concentrations below 1.0 mg/ml (Prylutska et al. 2007).
The male mice of C57Bl/6J line (20–21 g weight) were kept in a vivarium on a standard diet. The average temperature in a vivarium was 20 ± 1°C. We have to mention that in all experiments performed in the present work, we followed the international principles of European Convention for protection of vertebrate animals.
Tumor transplantation (Lewis lung carcinoma) was performed by intramuscular injection to the animal’s limb (initial number of tumor cells was equal to ∼5 × 105; antitumor effect) or to the pad of animal’s limb (initial number of tumor cells was equal to ∼1 × 106; antimetastatic effect). As is known, the strain of this tumor is characterized by a high degree of lung metastases damage.
The C60FAS in the volume of 0.1 ml (the initial concentration of C60 fullerenes in water was 1.0 mg/ml) was injected intraperitoneal to the animals before the transplantation of tumor (group 1; protective effect) one time for 5 days with interval through a day. Injection of C60FAS (0.1 ml) in group 2 (inhibitive effect) was started in a day after the transplantation of tumor, which visually appeared on the 10th day. The C60FAS was injected one time for 5 days with interval through a day given the fact that C60 fullerenes, introduced intraperitoneal to rats (dose 500 mg/kg), are excreted from the body within 2–4 days (Gharbi et al. 2005). Finally, group 0 (mice with transplanted tumor without C60FAS injection) was used as control. The initial number of animals in each group was 5 in experiment 1, 7 in experiment 2 (antitumor effect) and also 7 in experiment 3 (antimetastatic effect). On the 20th day of experiment 3, all animals were put to death for the purpose of calculating the number of metastases in the lung of each animal. It is also important to note that these experiments were started in different time periods: experiment 1—middle of autumn 2010; experiment 2—end of winter 2011; and experiment 3—end of spring 2011.
The antitumor effectiveness of the applied technique was estimated by following quantitative indicators:
-
tumor growth inhibition (TGI, percent):
 , where V0 and V
i
are the average values of tumor volume in animals of group 0 (control) and experimental group i, respectively;
, where V0 and V
i
are the average values of tumor volume in animals of group 0 (control) and experimental group i, respectively; 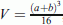 , where a and b are the length and width (in millimeters) of the tumor site;
, where a and b are the length and width (in millimeters) of the tumor site; -
increasing of animal life (IAL, percent):
 , where t0 and t
i
are the average life span of animals (in days) in group 0 (control) and experimental group i, respectively;
, where t0 and t
i
are the average life span of animals (in days) in group 0 (control) and experimental group i, respectively; -
survival of animals of experimental group i compared with control (
 , percent);
, percent); -
metastasis inhibition index (MII, percent):
 , where A0 and A1 are frequency of metastasis in the group 0 (control) and experimental group 1, respectively; B0 and B1 are the average number of metastases in certain organ of animals in group 0 (control) and experimental group 1, respectively.
, where A0 and A1 are frequency of metastasis in the group 0 (control) and experimental group 1, respectively; B0 and B1 are the average number of metastases in certain organ of animals in group 0 (control) and experimental group 1, respectively.
Within the histological studies, the tumor was fixed in 4% paraformaldehyde solution in phosphate buffer pH 7.4, dehydrated in alcohols of increasing concentration and poured in paraplast of U.S. production (Richard-Allan Scientific). The microscopic sections (thickness of 5 μm) were obtained using the rotary microtome (Leaica RM 21225 RT, Germany) and colored by Erlich hematoxylin and eosin. These sections were studied using the light optical microscope (Olympus BX51, Japan). The average number of mitotic and apoptotic cells, and the mitotic and apoptotic indices were determined by counting ten regions of view (each of them contained ∼500 cells) with magnification 400×. The percentage of area occupied by necrosis was estimated using the “Olympus DP-Soft” morphometric software.
The statistical analysis of obtained results was performed using STATISTICA software package including numerical methods (Gubler 1978).
3 Results and discussion
The STM images of submonolayer C60 fullerene film deposited from aqueous solution (C60 fullerene concentration in water was 1.0 mg/ml) on Au(111) surface are shown in Fig. 1. The images reveal almost random arrangement of C60 fullerene clusters with sizes up to ∼2.8 nm (the first stable sphere-like cluster consisting of 13 hydrated C60 fullerenes (Prilutsky et al. 1999; Bulavin et al. 2000) (Fig. 1a). Despite the high mobility of C60 molecules on Au(111) at the room temperature, images of single C60 molecules were also made (Fig. 1b).
a Large-scale STM image of submonolayer C60 fullerene film deposited from aqueous solution (C60 fullerene concentration in water was 1 mg/ml) on Au(111) surface. Some C60 fullerene clusters are aligned along preferential direction <112>. b Single C60 fullerenes. Lateral size is increased because of shape of the tip. Inset: cross-section along line AB. Scanning parameters: It = 40 pA, Ut = 0.7 V
The results extracted from experiment 1 (antitumor effect) are presented in Table 1. Antitumor effect of C60 fullerenes was recorded on the 15th day after transplantation of tumor in group 1 (protective effect; 25th day after the introduction of C60 fullerenes) and in group 2 (inhibitive effect). Inhibition of tumor growth in group 1 shows a tendency to decrease from 17.0% (15th day) to 3.1% (25th day after tumor transplantation). Inhibition of tumor growth in group 2 increased from 30.0% (15th day), reached a maximum of 37.2% (18th day) and then decreased to 16.0% (25th day after tumor transplantation). The last animal in the control group 0 died on the 32th day of the experiment. The survival of animals in groups 1 and 2 was 20.0% and 40.0%, respectively, compared to the control. Finally, the life duration for last animal in group 1 (protective effect) and group 2 (inhibitive effect) (38 days) was about 1.2 times higher compared to the control (31 days).
The results obtained from experiment 2 (antitumor effect) are presented in Table 2. Antitumor effect of C60 fullerenes was recorded on the 13th day after transplantation of tumor in group 1 (protective effect; 23th day after the introduction of C60 fullerenes) and in group 2 (inhibitive effect). Inhibition of tumor growth in group 1 shows a tendency to increase (in contrast to the results of experiment 1) from 15.0% (13th day) to the maximum value of 35.0% (27th day after tumor transplantation). Inhibition of tumor growth in group 2 reached a maximum of 25.1% (16th day) and then decreased to 5.0% (27th day after tumor transplantation). The last animal in the control group 0 died on the 30th day of the experiment. The survival of animals in groups 1 and 2 was 28.6% and 85.7%, respectively, compared to the control. These values were higher compared with those obtained in experiment 1. The extension of animal life in group 1 was found to be 30.3% while in group 2 was estimated to be 21.8% compared to the control. Finally, the life duration for last animal in group 1 (53 days) (protective effect) and group 2 (36 days) (inhibitive effect) was accordingly ∼1.8 and ∼1.2 times greater compared to the control (29 days) (this value agrees well with a same for group 2 in experiment 1).
For visual comparison of tumor growth, Fig. 2 presents the animals in group 2 (inhibitive effect; Fig. 2a) and group 1 (protective effect; Fig. 2b) on 34th day after tumor transplantation. Estimations demonstrate that the average tumor volume in experimental group 2 (inhibitive effect) is about ∼1.7 times greater compared to that in group 1 (protective effect).
It is also important to note that the visual observation of experimental animals revealed that animals in group 1 (protective effect) compared to the animals in group 2 (inhibitive effect) have greater mobility and that the color of their hair was more explicit shade. Finally, the life duration for last animal in group 1 (protective effect) seems to be about ∼1.5 times greater compared to that of last animals in group 2 (inhibitive effect).
The results obtained from experiment 3 (antimetastatic effect) clearly demonstrate that the metastasis inhibition index for group 1 (protective effect)  is twice the value of this index for group 2 (inhibitive effect)
is twice the value of this index for group 2 (inhibitive effect)  on the 20th day after tumor transplantation.
on the 20th day after tumor transplantation.
Figure 3 presents the histological data for the animal tumors of group 0 (control; Fig. 3a), group 1 (protective effect; Fig. 3b) and group 2 (inhibitive effect; Fig. 3c) on the 20th day after tumor transplantation in experiment 3 (antimetastatic effect).
Histological sections of Lewis lung carcinoma in male mice of C57Bl/6J line on the 20th day after tumor transplantation (Experiment 3 (antimetastatic effect): start of tumor transplantation—20 May 2011. Start of C60FAS injection after tumor transplantation—22 May 2011): a (control)—small areas of necrosis of tumor cells, apoptosis (200×); b (group 1; protective effect)—mitosis and apoptosis of tumor cells (400×); c (group 2; inhibitive effect)—areas of necrosis of tumor cells (200×)
Lewis lung carcinoma consists of polymorphic cells, most of which are occupied by the nucleus with large nucleolus, condensed chromatin and light areas of karyoplasm. Mitosis, apoptosis and necrosis of tumor cells are observed in all groups (Fig. 3). The isolated lymphocytes are presented in the tumor stroma, and infiltration of polymorphonuclear leukocytes is observed. Morphometric studies show the differences in the area of necrosis, mitotic and apoptotic indices between the study group and control, namely: area of necrosis in tumors was 17.7% (control), 12.7% (group 1; protective effect) and 8.3% (group 2; inhibitive effect); the mitotic index was 1.57 (control), 1.20 (group 1; protective effect) and 1.82 (group 2; inhibitive effect); the apoptotic index was 2.0 (control), 4.7 (group 1; protective effect) and 2.9 (group 2; inhibitive effect). As can be seen, the area in the tumor necrosis and apoptotic index value for group 1 (protective effect) exceed these parameters for group 2 (inhibitive effect). Conversely, the value of mitotic index for group 1 (protective effect) is less than this parameter for group 2 (inhibitive effect). Thus, C60 fullerenes, which were injected to animals before the tumor transplantation (protective effect), activate processes of necrosis and apoptosis of tumor cells, and C60 fullerenes, which are injected to animals after the tumor transplantation (inhibitive effect), activate the process of mitosis of tumor cells.
4 Conclusion
C60FAS, without showing direct cytotoxicity, cause at low single therapeutic dose (5 mg/kg) antitumor immune response. Specifically, it was observed that a treatment with C60FAS inhibits tumor volume of Lewis lung carcinoma in male mice of C57Bl/6J line. Namely, within the study of protective effect of C60FAS, it was found that the maximum therapeutic effect was reached 35.0% for the index of tumor growth inhibition. The survival of animals was 28.6% compared to the control. An extension of animal life of about 30.3% was found. Within the study of inhibitive effect of C60FAS, the treatment purposefully suppresses the growth of this tumor: the maximum therapeutic effect reached 25.1% for the index of tumor growth inhibition; the survival of animals was 85.7% compared to the control; and an extension of animal life of about 21.8% was found. The metastasis inhibition index was 96% for protective effect and 48% for inhibitive effect of C60FAS. Finally, the anticancer effect of C60FAS was confirmed by histological data. The obtained results can be explained as a result of the high antioxidant activity of C60 fullerenes, neutralizing excess reactive oxygen species in the cell and possibly blocking the specific cell receptors, for example, endothelial growth factor. The proposed treatment with C60FAS can be very promising in clinical oncology for the inhibition of tumor growth (Prylutska et al. 2011).
References
Andrievsky G, Klochkov V, Derevyanchenko L (2005) Is the C60 fullerene molecule toxic?! Fullerenes, Nanotubes, and Carbon Nanostruct 13:363–376
Boyle P, Levin B (2008) World cancer report. WHO, Lyon
Bulavin L, Adamenko I, Prylutskyy Yu, Durov S, Graja A, Bogucki A, Scharff P (2000) Structure of fullerene C60 in aqueous solution. Phys Chem Chem Phys 2:1627–1629
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438:932–936
Cataldo F, Da Ros T (eds) (2008) Medicinal chemistry and pharmacological potential of fullerenes and carbon nanotubes. Series: Carbon materials: chemistry and physics. Springer, Netherlands
Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, Seta P, Larroque Ch (2002) Cellular localisation of a water-soluble fullerene derivative. Biochem Biophys Res Commun 294:116–119
Freitas RA Jr (2003) Nanomedicine, volume IIA: biocompatibility. Landes Bioscience, Georgetown
Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F (2005) C60 fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett 5:2578–2585
Gubler EV (1978) Computational methods of analysis and recognition of pathological processes. Medicine, Moscow
Johnston HJ, Hutchison GR, Christensen FM, Aschberger K, Stone V (2010) The biological mechanisms and physicochemical characteristics responsible for driving fullerene toxicity. Toxicol Sci 114:162–182
Meng H, Xing G, Sun B, Zhao F, Lei H, Li W et al (2010) Potent angiogenesis inhibition by the particulate form of fullerene derivatives. ASC Nano 4:2773–2783
Murugesan S, Mousa SA, O’Connor LJ, Lincoln DW, Linhardt RJ (2007) Carbon inhibits vascular endothelial growth factor- and fibroblast growth factor-promoted angiogenesis. FEBS Lett 581:1157–1160
Prylutska SV, Matyshevska OP, Golub AA, Prylutskyy YuI, Potebnya GP, Ritter U, Scharff P (2007) Study of C60 fullerenes and C60-containing composites cytotoxicity in vitro. Mater Sci Engineer C 27:1121–1124
Prylutska SV, Grynyuk II, Matyshevska OP, Prylutskyy YuI, Ritter U, Scharff P (2008) Anti-oxidant properties of C60 fullerenes in vitro. Fullerenes, Nanotubes, and Carbon Nanostruct 16:698–705
Prylutska SV, Burlaka AP, Prylutskyy YuI (2011) Application of unmodified C60 fullerenes as anticancer agents in the therapy of malignant tumors. Patent of Ukraine for an invention N a201104882, 14 April 2011
Prilutsky YuI, Durov SS, Yashchuk VN, Ogul’chansky TYu, Pogorelov VE, Astashkin YuA, Buzaneva EV, Kirghizov YuD, Andrievsky GV, Scharff P (1999) Theoretical predictions and experimental studies of self-organization C60 nanoparticles in water solution and on the support. Eur Phys J D 9:341–343
Scharff P, Risch K, Carta-Abelmann L, Dmytruk IM, Bilyi MM, Golub OA, Khavryuchenko AV, Buzaneva EV, Aksenov VL, Avdeev MV, Prylutskyy YuI, Durov SS (2004) Structure of C60 fullerene in water: spectroscopic data. Carbon 42:1203–1206
Turov VV, Chehun VF, Krupskaya TV, Barvinchenko VN, Chehun SV, Ugnichenko AP, Prylutskyy YuI, Scharff P, Ritter U (2010) Effect of small addition of C60 fullerenes on the hydrated properties of nanocomposites based on highly dispersed silica and DNA. Chem Phys Lett 496:152–156
Yin JJ, Lao F, Fu PP, Zhao Y, Xing G, Gao X et al (2008) Inhibition of tumor growth by endohedral metallofullerenol nanoparticles optimized as reactive oxygen species scavenger. Mol Pharmacol 74:1132–1140
Yin JJ, Lao F, Fu PP, Wamer WG, Zhao Y, Wang PC et al (2009) The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials 30:611–621
Acknowledgments
This work was partly supported by BMBF grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Prylutska, S.V., Burlaka, A.P., Klymenko, P.P. et al. Using water-soluble C60 fullerenes in anticancer therapy. Cancer Nano 2, 105–110 (2011). https://doi.org/10.1007/s12645-011-0020-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12645-011-0020-x
 , where V0 and V
i
are the average values of tumor volume in animals of group 0 (control) and experimental group i, respectively;
, where V0 and V
i
are the average values of tumor volume in animals of group 0 (control) and experimental group i, respectively; 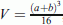 , where a and b are the length and width (in millimeters) of the tumor site;
, where a and b are the length and width (in millimeters) of the tumor site; , where t0 and t
i
are the average life span of animals (in days) in group 0 (control) and experimental group i, respectively;
, where t0 and t
i
are the average life span of animals (in days) in group 0 (control) and experimental group i, respectively; , percent);
, percent); , where A0 and A1 are frequency of metastasis in the group 0 (control) and experimental group 1, respectively; B0 and B1 are the average number of metastases in certain organ of animals in group 0 (control) and experimental group 1, respectively.
, where A0 and A1 are frequency of metastasis in the group 0 (control) and experimental group 1, respectively; B0 and B1 are the average number of metastases in certain organ of animals in group 0 (control) and experimental group 1, respectively.

