Abstract
Farming of Eucheuma denticulatum is a major activity in Zanzibar affecting seagrass ecosystems primarily through shading and trampling. The aim of this study was to test the impacts of shading and trampling during seaweed farming on seagrass meadows composed by Halophila stipulacea and Thalassia hemprichii and their associated benthic macroalgae. Areas covered by these species were selected for the building of seaweed farms in three treatments: seaweed farm plots (with shading and trampling effects), trampling plots (with trampling effects only), and control plots (with no shading or trampling effects). Reduction of light within the plots was recorded over 9 weeks. Percentage cover of seagrasses and macroalgae and shoot density of seagrasses were measured over 12 weeks to assess the impact of shading and trampling by seaweed farming activities. Light was significantly reduced in the seaweed farm plots by 75 to 90% by the end of a seaweed growth cycle. H. stipulacea, despite its capacity for rapid growth, was significantly affected by the combination of shading and trampling under the seaweed farm treatment, while the climax seagrass species T. hemprichii was unaffected. Due to the decline in H. stipulacea, benthic macroalgae cover increased in the seaweed farm treatment, suggesting a change in seagrass community dynamics. In contrast, trampling had a negative effect on the benthic macroalgae as an isolated disturbance, which suggests that seagrasses are more resistant to trampling than macroalgae and would likely dominate the benthic macrophyte community under these conditions.
Similar content being viewed by others
Introduction
Farming of red macroalgae of the genus Eucheuma is a major economic activity in Asian countries including the Philippines and Indonesia (Hurtado et al. 2014). In 2016, the total production of Eucheuma worldwide was around 10,519 thousand tons of live weight (FAO 2018), of which 214 thousand tons were from the species Eucheuma denticulatum. The value of this seaweed comes from its carrageenan, a substance used for its gelling, thickening, emulsifying, and water holding properties (Juanich 1988).
The success of seaweed farming stems from its low cost and simple infrastructure needs relative to its high economic benefit. Seaweed farming introduction has been promoted in coastal communities of developing countries as an alternative livelihood to fishing (Smith 1979). In Zanzibar, Tanzania, the adoption of seaweed farming was highly successful (Crawford et al. 2002), improving the household economy in comparison to previous situations in which seaweed farming was not practiced (Msuya 2006). Several villages in Zanzibar adopted the farming of the red macroalgae Eucheuma denticulatum around 1989 (Pettersson-Löfquist 1995; Msuya 2011), with a production of 12,000 metric tons of dry weight of this seaweed in 2010 (de San 2012). By 2008, seaweed became a leading contributor to the economy of Zanzibar as the largest marine export product from Tanzania (Eklöf et al. 2012).
The most popular cultivation method in Zanzibar is the off-bottom monoline deployed in the intertidal area. Most of the farms are located on sandy bottoms or seagrass meadows following the recommendations from the Food and Agriculture Organization of the United Nations (Juanich 1988), in which it is encouraged to choose sites where marine plants are already growing. Seaweed farming is considered a sustainable and environmentally friendly aquaculture activity as no chemicals or fertilizers are used and no waste is produced (Bryceson 2002). Nevertheless, the expansion of farming along the coasts of tropical countries combined with weak or non-existent regulations (de la Torre-Castro 2012; Hedberg et al. 2018) makes it necessary to assess more closely the potential environmental impacts it may have on natural coastal habitats. Seagrass meadows, in particular, occupy the same physical space as seaweed farms due to the FAO recommendations and convenience for the seaweed farmers (de la Torre-Castro and Rönnbäck 2004; Hedberg et al. 2018).
Seagrass are angiosperms that grow in the marine environment. They are one of the most important coastal ecosystems due to a number of ecosystem services they provide (Costanza et al. 1997), such as climate regulation (Duarte and Chiscano 1999), nutrient filtering (Hemminga et al. 1991), and habitat provision (Orth et al. 1984). Effects of seaweed farming on seagrass ecosystems include lower seagrass shoot density, cover, biomass and canopy height, and indirect effects on macrofauna biomass relative to unfarmed areas (Eklöf et al. 2005). More specifically, the biomass of the seagrass species Thalassia hemprichii was shown to be lower in areas with seaweed farms than without seaweed farms (Lyimo et al. 2006). Another study in Zanzibar (Eklöf et al. 2006) showed that a larger climax species like Enhalus acoroides is more negatively affected than the smaller climax species T. hemprichii with greater impacts on above-ground biomass, shoot density, and length. Seaweed farming was also recently reported to be one of the leading causes for the decrease in seagrass cover in Indonesia (Unsworth et al. 2018). Apart from the effects of shading, trampling has been reported to negatively impact seagrass meadows, algae dominated communities, and macrobenthos (Eckrich and Holmquist 2000; Milazzo et al. 2004; Alexandre et al. 2005). A study testing the effects of trampling in combination with shading in seaweed farms on seagrass ecosystems showed that shading was mainly responsible for negative effects on climax seagrass species, while trampling had inconsistent effects (Blankenhorn 2007).
These studies challenge the notion that seaweed farming activities are sustainable and harmless to the natural seagrass habitat and lead to further questions as to the main driver of seagrass decline under extensive seaweed farming. The studies so far have focused uniquely on the effects of seaweed farming on climax species. Less research, however, has been done on seagrass community dynamics, which may also shift under seaweed farming pressure. In particular, changes in abundance of opportunistic and ephemeral species, such as Halophila stipulacea, may lead to changes in ecosystem function, as has been shown in bioregions where it is invasive and has successfully displaced native species (Ruiz and Ballantine 2004; Willette and Ambrose 2009; Willette and Ambrose 2012; Viana et al. 2019). H. stipulacea is native in East Africa (Lipkin 1975); it is therefore necessary to study if H. stipulacea could displace climax species where they are growing together under intense seaweed farming pressure.
For these reasons, we tested the effects of seaweed farming on a climax seagrass species (T. hemprichii), an opportunistic seagrass species (H. stipulacea) and their associated benthic macroalgae. We focused on the impacts of seaweed farms through their combined effects of shading and trampling, and on the impact of trampling alone as an isolated stressor. Additionally, we were interested in studying the temporal trend in relative abundance of these organisms under seaweed farming pressure as few studies have tested this disturbance over time. To test these effects, we built a seaweed farm plot, a trampling plot, and a control plot in three replicate experimental blocks within an extended area dominated by T. hemprichii, but also with the presence of H. stipulacea and benthic algae in Chwaka Bay (Zanzibar, Tanzania). The seaweed farm plots exerted both shading and trampling pressure, the trampling plots exerted only trampling pressure, and the control plots exerted no disturbances. To measure the effects of the treatments on these organisms across time, we sampled the abundance of seagrass and total macroalgae approximately every 15 days for a total experimental duration of 96 days (25.11.2015–29.02.2016). We also sampled light reduction under seaweed farm plots relative to unfarmed plots to measure the magnitude of the shading by the farms.
We hypothesized that T. hemprichii would show a negative trend under seaweed farming and trampling pressure, while benthic macroalgae and H. stipulacea would be less affected. This could, therefore, lead to a shift from a seagrass community dominated by a climax seagrass species to opportunistic seagrass and benthic macroalgae species, which may radically change the ecosystem structure and function.
Materials and methods
Study site
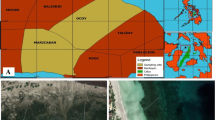
Chwaka Bay (Fig. 1a) is located in the east coast of Zanzibar. It is surrounded by mangroves in the south and sandy beaches and coral rubble on the west and east sides. Several channels run from the mangrove area to the mouth of the bay. Extensive seagrass meadows mixed with macroalgae cover the bay, together with bare carbonate sand and coral rock and rubble. The seagrass meadows in the selected site (− 6.1489°, 39.4528°) were dominated by Thalassia hemprichii (Ehrenberg) Ascherson 1871, mixed with Halophila stipulacea (Forsskål) Ascherson in Anon. 1868. The macroalgae Valonia sp. C.Agardh 1823, Halimeda sp. J.V.Lamouroux 1812, nom. et typ. cons., and Caulerpa sp. J.V.Lamouroux 1809 were also present in the study site mixed with the seagrass.
a Map of the study area along the east coast of Unguja Island, Zanzibar (small box with inset of Chwaka Bay blown up). Chwaka Bay is surrounded by several villages, Chwaka being the biggest one in the area. Several hotels are present between Chwaka and Marumbi, the two villages closest to the study area where the seaweed farm experimental plots were built. Map generated with Quatum GIS (Version 2.18.3). b Schematic representation of the treatment plots in the experiment. Figure produced with InkScape (Version 0.92.2)
Chwaka Bay is surrounded by several villages which depend on fishing as their main economic activity, with seaweed farming as a secondary source of income. Seaweed farms are present in the west coast of the bay, in shallow waters, and close to the coastal areas, mainly over seagrass meadows but also on sandy bottoms. During the experimental time, the temperature of the seawater was 1.9 ± 0.3 °C, the conductivity 52.9 ± 0.3 μS/m, and the pH 8.46 ± 0.1 (averages ± standard errors).
Seaweed farm experimental plots
Two local seaweed farmers provided the material and instructed on how to build the farms in the same method as they do in Chwaka Bay. We delimited three blocks (A, B, and C, separated by approximately 50 m) in an area dominated by T. hemprichii with presence of H. stipulacea and a mixed macroalgae community. We built three treatment plots (each 3 m wide and 4 m long, with an area of 12 m2) nested within each block: one seaweed farm plot, one trampling plot, and one control plot (each separated by 3–5 m within a block, Fig. 1b), resulting in a total of three replicate plots per treatment.
The seaweed farm consisted of four seaweed lines (ropes) tied to eight wooden pegs (two for each line), as is done in the off-bottom method. In each of the ropes, twelve E. denticulatum seedlings were attached by using the “tie-tie” method with a total of 48 seedlings per farm. The seedling size was standardized based on typical farming practices for E. denticulatum used at the beginning of the harvesting cycle. The trampling plot consisted of the same line structure as the seaweed farm plot, but without E. denticulatum attached to the ropes. The control plot consisted of four wooden pegs pressed into the sediment within the seagrass meadow, delimiting an area of the same size as the seaweed farm plot.
The experiment ran for 96 days (25.11.2015–29.02.2016) and was sampled approximately every 15 days for a total of seven sampling times (days 0, 15, 34, 49, 63, 81, and 96). The experiment was carried out during the Kaskazi season, which is generally considered unfavorable for seaweed growth (Hassan and Othman 2019). Nevertheless, the seaweeds grew uniformly and no die-off or problems with seaweed growth were detected (personal observation). The seaweed was not harvested until the end of the experiment. In every sampling, the seaweed and the seaweed farm ropes were cleaned from epiphytes, debris, and sediment by carefully shaking the algae to remove sediment and loose epiphytes and hand picking any epiphytic algae and anemones growing on the algae or on the ropes. Loose epiphytes were then flushed by the tidal current or directly thrown out of the plots.
Trampling pressure was exerted by one scientist walking between the lines in the seaweed farm and trampling plots during the sampling and cleaning time. The total time in which trampling was exerted in each seaweed farm and trampling plot was of about 40 min per sampling campaign. There was no trampling between samplings in order to reproduce the same effect that actual seaweed farmers would have on their farms. At the end of the experiment, a total of 4 h and 40 min of trampling was exerted in each seaweed farm and trampling plot by one person. With this design, shading and trampling were applied in the seaweed farm plots, trampling on the trampling plots, and no-disturbance on the control plots.
Seagrass and benthic macroalgae variables
Percentage cover and shoot density of seagrasses were measured taking three random points in each plot at each sampling time with 0.25 m2 and 0.01 m2 quadrats, respectively. Percentage cover was included as the only variable for the measurement of benthic macroalgae as macroalgae could not be assessed by shoot density. The Braun-Blanquet scale was used for the cover measurements (Mueller-Dombios and Ellenberg 1974), consisting of a scale of 8 numbers each one referring to an interval of percentage cover. The scale goes as follows: 0.1 = < 5% solitary, 0.5 = < 5% sparse, 1 = < 5% numerous, 2 = cover ≥ 5% - ≤ 25%, 3 = cover > 25% - ≤ 50%, 4 = cover > 50 - ≤ 75%, and 5 = cover > 75%. The cover categories were transformed into the midpoint cover range (Braun-Blanquet 1964). The percentage cover was then divided by 100, to obtain proportions between 0 and 1. Shoot density was measured counting the individual shoots of the two seagrass species within the 0.01 m2 quadrat.
Light reduction
A Li-Cor 1500 with a PAR (photosynthetically active radiation) sensor was used to measure the degree of shading by the seaweed growing over the seagrass meadow at each sampling time. Three random points were chosen within the seaweed farm plots and outside the farmed plots. The underwater PAR sensor (μmol photons m−2 s−1) was placed just below the water surface and at the seagrass canopy, while recording also the water depth at the canopy. Light reduction was calculated as the percent difference in surface and bottom PAR irradiance for the seaweed farm plots and the control plots. The worksheets with PAR data in three samplings were unfortunately lost (days 49, 81, and 96); therefore, we present only the data from the days 0, 15, 24, and 63 (up to 9 weeks of the experiment). Nevertheless, as the typical harvesting cycle in Zanzibar is between 35 and 45 days (Hurtado et al. 2017), these values are representative of the light reduction experienced by seagrass and its associated community due to seaweed farming.
Statistical analysis
Statistical software R (R Core Team 2016) was used for the statistical analysis. Graphs were plotted in R using the package “ggplot2” (Wickham 2016) and “ggpubr” (Kassambara 2020). Aesthetical editing of the figures was done using InkScape (Version 0.92.2).
To test whether there was a change in shoot density and cover in time due to shading and/or trampling, the slopes of regression models were compared. Time, treatment, and their interaction (slope) were tested as fixed effects. Due to the use of the experimental block design, block and its interaction with both sampling time and treatment were included as fixed effects. Due to the added complication of including extra interactions with block, analysis of deviance tests and likelihood ratio tests were used to simplify the models if the interactions with block did not add explanatory power. Both treatment and sampling and their interaction were never dropped from the model as they were part of the hypothesis testing. Block was never dropped from the model to avoid pseudoreplication (see Supplemental Material for further details).
The differences in light reduction inside and outside of the seaweed farms (introduced in the model as “area”) at each sampling time were analyzed using a linear model. Block and its interaction with the sampling area (outside or inside the seaweed farms) were added as a covariate to avoid pseudoreplication. Analysis of deviance test was used for model comparison and simplification. The significance in the differences in light reduction inside and outside the seaweed farms was obtained with analysis of variance (Type II test). Another linear model was used for the analysis of the differences in light reduction within an area (inside or outside seaweed farms) among the sampling times. Block and its interaction with sampling area were included in the model as fixed effects. The significance in the differences in light reduction within a sampling area in time was obtained with analysis of variance (Type II test). Post hoc pairwise comparisons were tested by using permutations in the models.
Results
Light reduction
In seaweed farm plots, the range of light reaching the seagrass canopy was between 31 and 1117 μmol of photons m−2 s−1 with a mean of 237 μmol of photons m−2 s−1. Outside the farmed plots, the range was between 437 and 1674 μmol of photons m−2 s−1, with a mean of 951 μmol of photons m−2 s−1. Although there was a small reduction in light outside the farmed plots, 15–25%, light reduction was significantly greater inside the seaweed farm plots at every sampling time, stabilizing between 75 and 90% from day 15 onwards (Fig. 2, Table 1). Block was a significant factor affecting light levels on day 0 and 63, and its interaction with the sampling area was significant on days 34 and 63 (Table 1). These results suggest that the light reduction, despite being always greater inside seaweed farm plots, was also dependent on the block in the experimental design, and therefore not completely uniform within seaweed farms and outside of the seaweed farms. Overall, light reduction within seaweed farm plots significantly increased during the sampling time (F (3, 24) = 72.854, p=3.382∙10−12) in which every sampling time was significantly higher than day zero (day 0 – day 15: t = 8.800, p = 5.61∙10−9; day 0 – day 34: t = 8.191, p = 2.07∙10−8; day 0 – day 63: t = 9.531, p = 1.25∙10−9).
Shoot density of T. hemprichii and H. stipulacea
In general, shoot density of T. hemprichii was higher in all plots compared to H. stipulacea and differed in its response to seaweed farming treatment (Fig. 3a–c, g–i, Table 2). As shown by the negative slopes of the regressions over time, shoot density of T. hemprichii and H. stipulacea decreased in the seaweed farm treatment (β = − 0.0004 ± 0.002; β = − 0.006 ± 0.006). In contrast, the slopes of the regressions were positive for both the control (β = 0.0034 ± 0.001; β = 0.0072 ± 0.004) and the trampling treatments (β = 0.0014 ± 0.002; β = 0.0191 ± 0.010). These differences were not significantly different for T. hemprichii (χ2 = 3.1402, df = 2, p = 0.208), while they were for H. stipulacea (χ2 = 7.1823, df = 2, p = 0.027). The slopes of the regression of the control and trampling treatments for H. stipulacea were significantly higher in comparison to the slope of the seaweed farm treatment (z = − 2.11, p = 0.034; z = − 2.35, p = 0.018). There were no differences between control and trampling plots (z = 1.16, p = 0.243).
a–o Shoot density and cover regression plots for the three treatments and the three blocks for all the variables. For shoot density plots, the y axis is the number of shoots in a 0.1 m2 quadrat. For cover plots, the y axis is the cover between 0 and 1, 0 meaning absence and 1 meaning complete cover, or 100%. The x axis is the time (days) that the experiment was running. Each of the columns represents one treatment, and each row represents the macrophyte tested. The colors represent the blocks and the regression line for each of the replicates
For H. stipulacea, the interaction between block and treatment was significant when tested as fixed effects, showing that the response was variable between the replicates (Table 2). Treatment as a categorical fixed effect was significant for the two variables, indicating that the response was dependent on treatment overall, but variable in time, resulting in small differences between slopes of the regressions.
Cover
Variability was high in the response of seagrass cover across the treatments over time (Fig. 3d–f, j–l, Table 2). Treatment as a fixed effect was significant for all the variables, and block was significant for all except H. stipulacea (Table 2). This result showed that, despite differences in responses between treatments, the variability over time translated into small differences in slopes overall.
Benthic macroalgae cover showed significant differences between slopes (Fig. 3m–o; χ2 = 8.622, df = 2, p = 0.012). The trampling treatment had a negative slope (β = − 0.007 ± 0.004), while both control and seaweed farm treatments showed a positive slope (β = 0.0009 ± 0.003; β = 0.005 ± 0.004). This slope difference was only significant between the seaweed farm and the trampling treatment (z = − 2.903, p = 0.003). These trends resulted in an increase of macroalgae cover from 22.1 to 31.4% in seaweed farms and a decrease from 47.5 to 25.9% in the trampling treatment.
Discussion
The effect of seaweed farming due to shading and trampling on seagrass meadows and its associated benthic macroalgae
The effect of seaweed farming resulted in a loss of shoots of H. stipulacea. However, with the exception of the negative slope in shoot density of T. hemprichii, no clear effect of seaweed farming on this species was found. Our initial hypothesis was, therefore, partially rejected. Our results show that H. stipulacea was more sensitive to seaweed farming than T. hemprichii in the short term. Our findings are in agreement with an earlier study from Eklöf et al. (2006), in which T. hemprichii biomass and shoot density were unaffected after 11 weeks of seaweed farming. Nevertheless, in permanent seaweed farms, shoot density of T. hemprichii has been shown to be lower than in seagrass beds without seaweed farms (Eklöf et al. 2005; Lyimo et al. 2006). This suggests that over a longer period of time, T. hemprichii can be negatively affected by seaweed farming if conditions persist. In the case of H. stipulacea, there was no previous literature addressing its response to seaweed farming. The shading levels measured in this study inside and outside of seaweed farms were within the range of light irradiance of survival for both H. stipulacea (Sharon et al. 2011; Winters et al. 2020) and T. hemprichii (Collier et al. 2012), without showing negative effects on their physiology, morphology, or density. This indicates that it is the additional stress of trampling combined with shading that is likely responsible for the response of H. stipulacea. This is also supported by Blankenhorn (2007) who showed that trampling had negative effects on E. acoroides only in combination with shading and unfavorable seasonality, but had no impact as an isolated effect. The higher resistance of T. hemprichii was probably due to its higher carbohydrate reserve in the rhizome (Alcoverro et al. 2001; Longstaff and Dennison 1999) that are often lacking in opportunistic species like H. stipulacea. The higher energy reserves of T. hemprichii may be explained by this species investment in below-ground tissues, which also makes it more robust in structure and resistant to physical damage. H. stipulacea, in contrast, has a very fragile morphology (small and thin leaves, thin rhizome, and short roots) that can be easily damaged and uprooted. The combination of the lack of energy reserves and its fragility may explain the loss of this species over time (a decrease in shoot density by half during the experiment), suggesting that this species could be displaced in the long term.
Cover is a visual indicator measured in a coarse scale, which was less accurate a measure than shoot counts and was not able to capture a change in a small quantity of shoots over time. Despite the proved usefulness of cover as a tool to assess seagrass abundance over larger spatial scales, we would not recommend cover as a measurement to capture small changes in seagrass densities over shorter temporal scales. Benthic macroalgae abundance was measured only as cover, and its slope in the seaweed farm treatment was positive and did not differ from the control. Taking into account that the shoot density of H. stipulacea was reduced in the seaweed farm treatments, macroalgae may be able out-compete seagrass under seaweed farming. This result partially agrees with our initial hypothesis, suggesting that benthic macroalgae can out-compete H. stipulacea under seaweed farms, but not T. hemprichii. It is important to point out the limitations of this result as well. As we did not identify all the components of the benthic macroalgae community, this shift cannot be extrapolated, as the change in the community is contingent on the algae species present in the area. Importantly, the loss of seagrass in the long term may also negatively affect macroalgae, as it has been reported that the cover of macroalgae is lower in seaweed farms compared to non-seaweed farm beds (Eklöf et al. 2005). The loss of seagrass may lead to a lower chance of macroalgae settling in the carbonate sand substrate and, therefore, not allow for the formation of a permanent community. Ultimately, there was no shift in the dominant macrophyte in the seagrass and macroalgae community. This indicates that, in the short term, there is no concern with the permanent loss of climax seagrass species. However, the loss of seagrass biodiversity due to the displacement of H. stipulacea can have unexpected consequences in ecosystem functioning (Somerfield et al. 2002; Duffy 2006).
The effect of trampling
The trampling treatment had similar results as the control treatment for the seagrass shoot density, showing that low-intensity trampling as an isolated disturbance had no effects on seagrass. On the other hand, the cover of benthic macroalgae decreased. The pattern that macroalgae followed in the trampling treatment differed from that of the seaweed farm plots where it increased. The reason behind the loss of macroalgae could be that, as there is no loss of seagrass due to the lack of shading, it cannot out-compete seagrass for space. This is further confirmed by the fact that the slope of the regression of benthic macroalgae cover is only significantly lower in the trampling treatment when compared to the seaweed farm treatment. This agrees with a similar experiment (Blankenhorn 2007), which showed that trampling had no effects on seagrass as an isolated disturbance. We therefore reject the initial hypothesis that trampling as a disturbance could change the dominant macrophyte in the area.
The interpretation of these results regarding seagrass shoot density should be, however, conservative. The opposite result is normally reported: increased trampling results in the loss of seagrass biomass (Eckrich and Holmquist 2000) and cover and reduction of blade length (Travaille et al. 2015). Trampling may also affect seagrass-associated fauna. Other studies showed that epifauna were affected by intense trampling of seagrass in the past (Eckrich and Holmquist 2000; Skilleter et al. 2006). In other ecosystems like mudflats, the diversity and abundance of sessile organisms changed (Rossi et al. 2007). Apart from the direct physical effect on the benthos, trampling has the side effect of increasing the suspended sediment in the water column. This effect was considered of little importance due to fast flushing of the sediment by tidal currents in the area (Personal observation). Reports in the literature show that suspended sediment inputs over decadal timescales can reduce benthic macroalgae species richness (Shepherd et al. 2009), while their composition and abundance depend more on algae reproductive traits in the short term (Eriksson and Johansson 2005). Nevertheless, this is not the case for this experiment and for the practice of seaweed farming, in general, as sediment input is not continuous or persistent. We can make a conservative assumption that sediment suspension and turbidity are not driving factors affecting seagrass and benthic macroalgae communities under seaweed farming.
Even though we found no negative effects of trampling on seagrass during the experiment, this response may be subjected to the frequency of the trampling. The frequency at which the experimental seaweed farm and trampling plots were sampled was low, and, therefore, did not negatively affect the seagrass. As farmers tend to work also in the low spring tide due to the lack of swimming training (Farmers of Chwaka Bay, personal communication), trampling frequency and the associated impact on seagrass would likely also be minimized. Nevertheless, if the trampling frequency or the number of people trampling on the plot increased, this result could lead to higher impacts on the seagrass ecosystem.
Management recommendations and current state of seaweed farming in Zanzibar
To date, the scientific literature on the effects of seaweed farms on seagrass meadows generally concludes with a similar message that despite the recorded negative effects of farming on seagrass meadows, it is generally a preferable aquaculture activity when compared with more destructive ones, like shrimp farming (Eklöf et al. 2006). Here we argue the contrary that, as any activity developed in the natural environment, it should be managed in order to minimize its negative effects. These management recommendations should take into account the social and economic context of this activity in the region. First, seaweed farming has decreased in the last years in Chwaka Bay (seaweed farmers, personal communication) due to the low revenue obtained from this activity. The commercial viability of seaweed in Zanzibar is threatened by several key points, including low price of the seaweed, low organization among farmers, and low institutional support. A comprehensive work by Songwe et al. (2016) explains in detail each of these problems. As a consequence, there is little incentive for the professionalization and proper management of seaweed farming. Management recommendations such as a shift from the off-bottom method to the floating long-line, which does not disturb seagrass but requires more equipment and investment, may not be adopted if the activity does not also bring economic revenue.
In the last policy briefs produced by The Revolutionary Government of Zanzibar (2014) and the Ministry of Livestock and Fisheries Development of the United Republic of the Tanzania (2014), the promotion of seaweed farming by value addition techniques and the implementation of coastal management for seaweed farming were highlighted as being necessary to professionalize seaweed farming and reduce environmental impacts. From our findings, there are two main recommendations that could avoid negative long-lasting effects of seaweed farming on seagrass meadows: (1) rotating crops and (2) targeting of bare sediment areas for seaweed farms. The first recommendation was suggested by Eklöf et al. (2006) and Blankenhorn (2007), and we agree that it would decrease the potential impacts of long-term seaweed farming on seagrass ecosystems. The second recommendation contradicts the recommendations of the FAO (Juanich 1988) for the site selection. Although we agree that farmers should locate their farms in areas where macrophytes already grow as a proof that environmental conditions are suitable for photosynthetic organisms, they should avoid the placing of farms directly on top of seagrass meadows. Another potential solution is to locate the lines further apart from each other to allow for more light reaching the seagrass canopy. These recommendations could be easily implemented, do not require extra investment, and would also protect other livelihoods such as fishing, which is tightly linked to the health of seagrass ecosystems.
Conclusions
This study shows that seaweed farming, through the combined effects of trampling and shading, can negatively affect the ephemeral species H. stipulacea despite its capacity for rapid growth. On the other hand, T. hemprichii was mainly unaffected in the short term. Due to the decline in H. stipulacea, benthic macroalgae cover increased in the seaweed farm treatment, suggesting a possible change in the seagrass and macroalgae community under the impact of seaweed farming. Trampling as an isolated disturbance had only a negative effect on the benthic macroalgae, which suggests that, under this isolated disturbance, seagrass can out-compete the benthic macroalgae community.
This study further contributes to the analysis of the effects of seaweed farms, raising awareness about possible long term effects over the seagrass and benthic macroalgae communities if seaweed farming is not properly managed. The question of the sustainability of seaweed farming should not only be addressed from an ecological point of view, but jointly with social and economic considerations under a climate change scenario. Efforts in the professionalization and organization of seaweed farming would help the farmers to bring extra value to their activity, together with a stronger capacity for the negotiation and bargain of the price of their product, while supporting coastal efforts to make it more sustainable.
References
Agardh CA (1823) Species algarum rite cognitae: cum synonymis, differentiis specificis et descriptionibus succinctis. sumtibus E. Mavritii. Vol 1
Alcoverro T, Manzanera M, Romero J (2001) Annual metabolic carbon balance of the seagrass Posidonia oceanica: the importance of carbohydrate reserves. Mar Ecol Prog Ser 211:105–116. https://doi.org/10.3354/meps211105
Alexandre A, Santos R, Serrão E (2005) Effects of clam harvesting on sexual reproduction of the seagrass Zostera noltii. Mar Ecol Prog Ser 298:115–122. https://doi.org/10.3354/meps298115
Ascherson P (1868) Vorarbeiten zu einer Uebersicht der phanerogamen Meergewachse. Linnaea 35:152–208
Ascherson P (1871) Die geographische verbreitung der seegräser. Petermans Geographische Mittheilungen 17(7):241–248
Blankenhorn SU (2007) Seaweed farming and artisanal fisheries in an Indonesian seagrass bed. Dissertation, University of Bremen
Braun-Blanquet J (1964) Pflanzensoziologie, Grundzüge der Vegetationskunde. 3rd edn. Springer
Bryceson I (2002) Coastal aquaculture developments in Tanzania: sustainable and non-sustainable experiences. Western Indian Ocean J Mar Sci 1(1):1–10
Collier CJ, Waycott M, Ospina AG (2012) Responses of four Indo-West Pacific seagrass species to shading. Mar Pollut Bull 65(4–9):342–354. https://doi.org/10.1016/j.marpolbul.2011.06.017
Costanza R, d’Arge R, De Groot R, Farber S, Grasso M et al. (1997) The value of the world’s ecosystem services and natural capital. Nature 387(6630):253. https://doi.org/10.1038/387253a0
Crawford BR, Pollnac RB, Sukmara A, Tulungen JJ (2002) Community-based coastal resources management: an interim assessment of the Proyek Pesisir field site in Bentenan and Tumbak villages, North Sulawesi, Indonesia. Narragansett, RI: University of Rhode Island, Coastal Resources Center. Technical report TE–02/01–E. https://www.crc.uri.edu/download/2000_Crawford_Tech_R_Community_Based_CRM.pdf accessed 22 October 2020
de la Torre-Castro M (2012) Governance for sustainability: insights from marine resource use in a tropical setting in the Western Indian Ocean. Coast Manag 40(6):612–633. https://doi.org/10.1080/08920753.2012.727738
de la Torre-Castro MD, Rönnbäck P (2004) Links between humans and seagrasses—an example from tropical East Africa. Ocean & Coastal Management 47(7–8):361–387. https://doi.org/10.1016/j.ocecoaman.2004.07.005
de San M (2012) The farming of seaweeds. FAO Report SF/2012/28. http://www.fao.org/3/a-bl759e.pdf Accessed 22 October 2020
Duarte CM, Chiscano CL (1999) Seagrass biomass and production: a reassessment. Aquat Bot 65(1–4):159–174. https://doi.org/10.1016/s0304-3770(99)00038-8
Duffy JE (2006) Biodiversity and the functioning of seagrass ecosystems. Mar Ecol Prog Ser 311:233–250. https://doi.org/10.3354/meps311233
Eckrich CE, Holmquist JG (2000) Trampling in a seagrass assemblage: direct effects, response of associated fauna, and the role of substrate characteristics. Mar Ecol Prog Ser 201:199–209. https://doi.org/10.3354/meps201199
Eklöf JS, de la Torre-Castro M, Adelsköld L, Jiddawi NS, Kautsky N (2005) Differences in macrofaunal and seagrass assemblages in seagrass beds with and without seaweed farms. Estuar Coast Shelf Sci 63(3):385–396. https://doi.org/10.1016/j.ecss.2004.11.014
Eklöf JS, Henriksson R, Kautsky N (2006) Effects of tropical open-water seaweed farming on seagrass ecosystem structure and function. Mar Ecol Prog Ser 325:73–84. https://doi.org/10.3354/meps325073
Eklöf JS, Msuya FE, Lyimo TJ, Buriyo AS (2012) Seaweed farming in Chwaka Bay: a sustainable alternative in aquaculture. In: People, nature and research in Chwaka Bay. WIOMSA, Zanzibar, pp. 213–233
Eriksson BK, Johansson G (2005) Effects of sedimentation on macroalgae: species-specific responses are related to reproductive traits. Oecologia 143(3):438–448. https://doi.org/10.1007/s00442-004-1810-1
FAO (2018) The State of World Fisheries and Aquaculture 2018 - meeting the sustainable development goals. http://www.fao.org/3/i9540en/i9540en.pdf Accessed 22 October 2020
Hassan IH, Othman WJ (2019) Seaweed (Mwani) farming as an adaptation strategy to impacts of climate change and variability in Zanzibar. In: Climate change and coastal resources in Tanzania. Springer, Cham, pp. 53–68
Hedberg N, von Schreeb K, Charisiadou S, Jiddawi NS, Tedengren M et al (2018) Habitat preference for seaweed farming–a case study from Zanzibar, Tanzania. Ocean & Coastal Management 154:186–195. https://doi.org/10.1016/j.ocecoaman.2018.01.016
Hemminga MA, Harrison PG, Van Lent F (1991) The balance of nutrient losses and gains in seagrass meadows. Mar Ecol Prog Ser 28:85–96. https://doi.org/10.3354/meps071085
Hurtado AQ, Gerung GS, Yasir S, Critchley AT (2014) Cultivation of tropical red seaweeds in the BIMP-EAGA region. J Appl Phycol 26(2):707–718. https://doi.org/10.1007/s10811-013-0116-2
Hurtado AQ, Critchley AT, Neish I (2017) Tropical seaweed farming trends, problems and opportunities. Springer Publ. AG, New York
Juanich GL (1988) Manual on seaweed farming 1. Eucheuma spp. FAO publications. http://www.fao.org/3/ac416e/ac416e00.htm accessed 22 October 2020
Kassambara A (2020) ggpubr: ‘ggplot2’ based publication ready plots. R package version 0.4.0. https://CRAN.R-project.org/package=ggpubr Accessed 02 November 2020
Lamouroux JVF (1809) Exposition des caractères du genre Dictyota, et tableau des espèces qu’il renferme. Journal de botanique (Desvaux) 2:38–44
Lamouroux JVF (1812) Sur la classification des Polypiers coralligénes non entiérement pierreux. Nouv Bull Sci Soc Philom Paris 3:181–188
Lipkin Y (1975) Halophila stipulacea, a review of a successful immigration. Aquat Bot 1:203–215. https://doi.org/10.1016/0304-3770(75)90023-6
Longstaff BJ, Dennison WC (1999) Seagrass survival during pulsed turbidity events: the effects of light deprivation on the seagrasses Halodule pinifolia and Halophila ovalis. Aquat Bot 65(1–4):105–121. https://doi.org/10.1016/s0304-3770(99)00035-2
Lyimo TJ, Mvungi EF, Lugomela C, Björk M (2006) Seagrass biomass and productivity in seaweed and non-seaweed farming areas in the East Coast of Zanzibar. Western Indian Ocean Journal of Marine Science 5(2):141–152. https://doi.org/10.4314/wiojms.v5i2.28506
Milazzo M, Badalamenti F, Riggio S, Chemello R (2004) Patterns of algal recovery and small-scale effects of canopy removal as a result of human trampling on a Mediterranean rocky shallow community. Biol Conserv 117(2):191–202. https://doi.org/10.1016/s0006-3207(03)00292-1
Ministry of Livestock and Fisheries Development United Republic of Tanzania (2014) First South West Indian Ocean Fisheries Governance and shared growth project-SWIOfish, Environmental and Social Assessment (ESA) and Environmental and Social Management Framework (ESMF). http://documents1.worldbank.org/curated/ar/921011468003576936/pdf/E46250V30P132100Box385306B00PUBLIC0.pdf Accessed 23 October 2020
Msuya FE (2006) The impact of seaweed farming on the social and economic structure of seaweed farming communities in Zanzibar, Tanzania. In Critchley AT, Ohno M, Largo DB (eds.) World seaweed resources: an authoritative reference system. Amsterdam, ETI BioInformatics. http://196.44.162.10:8080/xmlui/handle/123456789/653 accessed 23 October 2020
Msuya FE (2011) The impact of seaweed farming on the socioeconomic status of coastal communities in Zanzibar, Tanzania. World Aquacult 42:45–48
Mueller-Dombios D, Ellenberg H (1974) Aims and methods of vegetation ecology. John Willey & Sons. Inc., New York
Orth RJ, Heck KL, van Montfrans J (1984) Faunal communities in seagrass beds: a review of the influence of plant structure and prey characteristics on predator-prey relationships. Estuaries 7(4):339–350. https://doi.org/10.2307/1351618
Pettersson-Löfquist P (1995) The development of open-water algae farming in Zanzibar: reflections on the socioeconomic impact. Ambio 24(7–8):487–491. JSTOR www.jstor.org/stable/4314394 Accessed 22 October 2020
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/ Accessed 02 November 2020
Rossi F, Forster RM, Montserrat F, Ponti M, Terlizzi A et al (2007) Human trampling as short-term disturbance on intertidal mudflats: effects on macrofauna biodiversity and population dynamics of bivalves. Mar Biol 151(6):2077–2090. https://doi.org/10.1007/s00227-007-0641-0
Ruiz H, Ballantine DL (2004) Occurrence of the seagrass Halophila stipulacea in the tropical West Atlantic. Bull Mar Sci 75(1):131–135
Sharon Y, Levitan O, Spungin D, Berman-Frank I, Beer S (2011) Photoacclimation of the seagrass Halophila stipulacea to the dim irradiance at its 48-meter depth limit. Limnol Oceanogr 56(1):357–362. https://doi.org/10.4319/lo.2011.56.1.0357
Shepherd SA, Watson JE, Womersley HBS, Carey JM (2009) Long-term changes in macroalgal assemblages after increased sedimentation and turbidity in Western Port, Victoria, Australia. Bot Mar 52(3):195–206. https://doi.org/10.1515/bot.2009.036
Skilleter GA, Cameron B, Zharikov Y, Boland D, McPhee DP (2006) Effects of physical disturbance on infaunal and epifaunal assemblages in subtropical, intertidal seagrass beds. Mar Ecol Prog Ser 308:61–78. https://doi.org/10.3354/meps308061
Smith IR (1979) Traditional fisheries development in the Philippines. ICLARM Newsletter http://pubs.iclarm.net/resource_centre/WF_1282.pdf Accessed 22 October 2020
Somerfield PJ, Yodnarasri S, Aryuthaka C (2002) Relationships between seagrass biodiversity and infaunal communities: implications for studies of biodiversity effects. Mar Ecol Prog Ser 237:97–109. https://doi.org/10.3354/meps237097
Songwe BA, Khamis SM, Khalfan MH, Msuya FE (2016) Commercial seaweed farming in Zanzibar coastal villages: potential for innovative and competitive economic growth. Journal of the Open University of Tanzania 22:1153–1173
The Revolutionary Government of Zanzibar (2014) Non-governmental NGOS (NGOs) Policy https://tile.loc.gov/storage-services/service/gdc/gdcovop/2019352552/2019352552.pdf Accessed 22 October 2020
Travaille KL, Salinas-de-León P, Bell JJ (2015) Indication of visitor trampling impacts on intertidal seagrass beds in a New Zealand marine reserve. Ocean & Coastal Management 114:145–150. https://doi.org/10.1016/j.ocecoaman.2015.06.002
Unsworth RK, Ambo-Rappe R, Jones BL, La Nafie YA, Irawan A et al (2018) Indonesia’s globally significant seagrass meadows are under widespread threat. Sci Total Environ 634:279–286. https://doi.org/10.1016/j.scitotenv.2018.03.315
Viana IG, Siriwardane-de Zoysa R, Willette DA, Gillis LG (2019) Exploring how non-native seagrass species could provide essential ecosystems services: a perspective on the highly invasive seagrass Halophila stipulacea in the Caribbean Sea. Biol Invasions 21(5):1461–1472. https://doi.org/10.1007/s10530-019-01924-y
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag New York. https://CRAN.R-project.org/package=ggplot2 Accessed 02 November 2020
Willette DA, Ambrose RF (2009) The distribution and expansion of the invasive seagrass Halophila stipulacea in Dominica, West Indies, with a preliminary report from St. Lucia. Aquatic Botany 91(3):137–142. https://doi.org/10.1016/j.aquabot.2009.04.001
Willette DA, Ambrose RF (2012) Effects of the invasive seagrass Halophila stipulacea on the native seagrass, Syringodium filiforme, and associated fish and epibiota communities in the Eastern Caribbean. Aquat Bot 103:74–82. https://doi.org/10.1016/j.aquabot.2012.06.007
Winters G, Beer S, Willette DA, Viana IG, Chiquillo KL et al (2020) The tropical seagrass Halophila stipulacea: reviewing what we know from its native and invasive habitats, alongside identifying knowledge gaps. Front Mar Sci 7:300. https://doi.org/10.3389/fmars.2020.00300
Acknowledgments
The authors thank the staff of the Institute of Marine Sciences (IMS) in Stone Town for their support both administratively and scientifically, specifically Mtumwa Mwadini for his advice and help.
We want to thank the people from Chwaka Village for their help. Specifically to Hakim Machano for his help in the building of the experimental plots. Also we want to thank his son Hassan Machano for his help in the sampling activities. We would like to thank Stephanie Helber for her help in building the seaweed farms.
Funding
Open Access funding enabled and organized by Projekt DEAL. Leibniz Centre for Tropical Marine Research Master Thesis grant and the PROMOS scholarship (Programm zur Steigerung der Mobilität von Studierenden deutscher Hochschulen, University of Bremen) awarded to Agustín Moreira Saporiti partially funded the field work. This project was developed under the SUTAS program (Sustainable Use of Tropical Aquatic Systems - funding to Dieuwke Hoeijmakers). The Seagrass and Macroalgal Community Dynamics and Performance under Environmental Change (SEAMAC) project (Deutsche Forschungsgemeinschaft, DFG, TE 1046/3-1) awarded to Mirta Teichberg partially funded this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dror Angel
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 16 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moreira-Saporiti, A., Hoeijmakers, D., Msuya, F.E. et al. Seaweed farming pressure affects seagrass and benthic macroalgae dynamics in Chwaka Bay (Zanzibar, Tanzania). Reg Environ Change 21, 11 (2021). https://doi.org/10.1007/s10113-020-01742-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10113-020-01742-2