Photosynthesis and Growth of Pennisetum centrasiaticum (C4) is Superior to Calamagrostis pseudophragmites (C3) during Drought and Recovery
Abstract
:1. Introduction
2. Results
2.1. Climatic Conditions and Water Status
2.2. Gas Exchange Characteristics
2.3. Chlorophyll Fluorescence Characteristics
2.4. Biomass Characteristics
3. Discussion
3.1. Photosynthetic Adjustment and Biomass Response to Drought and Recovery for Two Psammophytes
3.2. Differentiation of Photosynthetic Performance and Biomass to Drought and Rewatering between Two Psammophytes
4. Materials and Methods
4.1. Soil Moisture and Climatic Conditions
4.2. Leaf Gas Exchange Measurement
4.3. Chlorophyll Fluorescence Measurement
4.4. Analysis of the Chlorophyll Fluorescence Transients: JIP-test
4.5. Biomass Measurement
4.6. Statistic Analysis
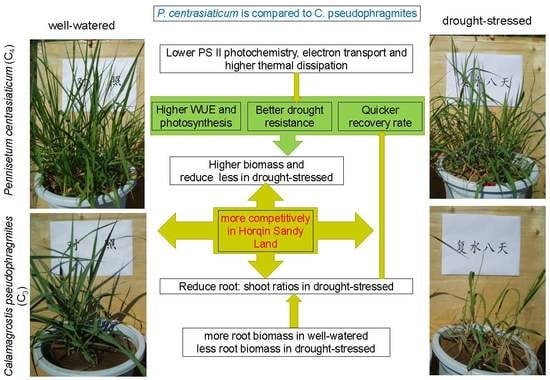
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koutroulis, A. Dryland changes under different levels of global warming. Sci. Total. Environ. 2019, 655, 482–511. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhao, X.; Zhou, R.; Zuo, X.; Zhang, J.; Li, Y. Physiological acclimation of two psammophytes to repeated soil drought and rewatering. Acta Physiol. Plant. 2011, 33, 79–91. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Shangguan, Z. A meta-analysis of leaf gas exchange and water status responses to drought. Sci. Rep. 2016, 6, 20917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Z.; Chen, Y.; Palta, J.A.; Prasad, P.V.V. Editorial: Adaptation of Dryland Plants to a Changing Environment. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and Metabolic Limitations to Photosynthesis under Drought and Salinity in C 3 Plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Flexas, J.; Diaz-Espejo, A.; Gago, J.; Gallé, A.; Galmés, J.; Gulías, J.; Medrano, H. Photosynthetic limitations in Mediterranean plants: A review. Environ. Exp. Bot. 2014, 103, 12–23. [Google Scholar] [CrossRef]
- Gallé, A.; Haldimann, P.; Feller, U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 2007, 174, 799–810. [Google Scholar] [CrossRef]
- Ghannoum, O. C4 photosynthesis and water stress. Ann. Bot. 2009, 103, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, E.; Gil, S.; Azcon-Bieto, J.; Nogues, S. The response of Arundo donax L. (C3) and Panicum virgatum (C4) to different stresses. Biomass Bioenergy 2016, 85, 335–345. [Google Scholar] [CrossRef]
- Kümpers, B.M.C.; Burgess, S.J.; Reyna-Llorens, I.; Smith-Unna, R.D.; Boursnell, C.; Hibberd, J.M. Shared characteristics underpinning C4 leaf maturation derived from analysis of multiple C3 and C4 species of Flaveria. J. Exp. Bot. 2017, 68, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Steinemann, S.; Zeng, Z.; McKay, A.; Heuer, S.; Langridge, P.; Huang, C.Y. Dynamic root responses to drought and rewatering in two wheat (Triticum aestivum) genotypes. Plant Soil 2015, 391, 139–152. [Google Scholar] [CrossRef]
- Yi, X.-P.; Zhang, Y.-L.; Yao, H.; Luo, H.-H.; Gou, L.; Chow, W.S.; Zhang, W.-F. Rapid recovery of photosynthetic rate following soil water deficit and re-watering in cotton plants (Gossypium herbaceum L.) is related to the stability of the photosystems. J. Plant Physiol. 2016, 194, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Hura, K.; Ostrowska, A.; Dziurka, K. Rapid plant rehydration initiates permanent and adverse changes in the photosynthetic apparatus of triticale. Plant Soil 2015, 397, 127–145. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.N.; Silveira, J.A.; Ribeiro, R.V.; Vieira, S.A. Photoprotective function of energy dissipation by thermal processes and photorespiratory mechanisms in Jatropha curcas plants during different intensities of drought and after recovery. Environ. Exp. Bot. 2015, 110, 36–45. [Google Scholar] [CrossRef]
- Wang, T.; Xue, X.; Zhou, L.; Guo, J. Combating Aeolian Desertification in Northern China. Land Degrad. Dev. 2015, 26, 118–132. [Google Scholar] [CrossRef]
- Luo, Y.; Zuo, X.; Li, Y.; Zhang, T.; Zhang, R.; Chen, J.; Lv, P.; Zhao, X. Community carbon and water exchange responses to warming and precipitation enhancement in sandy grassland along a restoration gradient. Ecol. Evol. 2019, 9, 10938–10949. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.Y.; Zhao, X.Y.; Qu, H.; Zuo, X.A.; Wang, S.K.; Huang, W.D.; Luo, Y.Q.; Chen, M. Photosynthetic performance and growth traits in Pennisetum centrasiaticum exposed to drought and rewatering under different soil nutrient regimes. Acta Phys. Plant. 2014, 36, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental Ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Cifre, J.; Escalona, J.M.; Galmés, J.; Gulías, J.; Lefi, E.-K.; Martínez-Cañellas, S.F.; Moreno, M.T.; Ribas-Carbo, M.; et al. Understanding down-regulation of photosynthesis under water stress: Future prospects and searching for physiological tools for irrigation management. Ann. Appl. Biol. 2004, 144, 273–283. [Google Scholar] [CrossRef]
- Adak, M.K. Analysis of Chlorophyll Fluorescence: A Reliable Technique in Determination of Stress on Plants. In Eco-Friendly Agro-Biological Techniques for Enhancing Crop Productivity; Springer: Singapore, 2018; pp. 63–88. [Google Scholar]
- Force, L.; Critchley, C.; Van Rensen, J.J.S. New fluorescence parameters for monitoring photosynthesis in plants. Photosynth. Res. 2003, 78, 17–33. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazar, D.; Kromdijk, J. Govindjee. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Strasser, B.J.; Strasser, R.J. Measuring fast fluorescence transients to address environmental questions: The JIP-Test. In Photosynthesis: From Light to Biosphere, Proceedings of the 10th International Photosynthesis Congress, Montpellier, France, 20–25 August 1995; Springer: Dordrecht, The Netherlands, 1995; Volume 5, pp. 977–980. [Google Scholar]
- Varone, L.; Ribas-Carbó, M.; Cardona, C.; Galle, A.; Medrano, H.; Gratani, L.; Flexas, J. Stomatal and non-stomatal limitations to photosynthesis in seedlings and saplings of Mediterranean species pre-conditioned and aged in nurseries: Different response to water stress. Environ. Exp. Bot. 2012, 75, 235–247. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmés, J.; Medrano, H.; Ribas-Carbo, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Ripley, B.; Frole, K.; Gilbert, M.E. Differences in drought sensitivities and photosynthetic limitations between co-occurring C3 and C4 (NADP-ME) Panicoid grasses. Ann. Bot. 2010, 105, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Fan, G.; Zhou, D.; Pang, J. Phenotypic plasticity of four Chenopodiaceae species with contrasting saline-sodic tolerance in response to increased salinity-sodicity. Ecol. Evol. 2019, 9, 1545–1553. [Google Scholar] [CrossRef] [Green Version]
- Sack, L.; Pasquet-Kok, J.; Taylor, S.; Scoffoni, C.; Christin, P.A.; Diener, A.; Edwards, E.J.; Osborne, C.P. Hyper-efficient water transport enables the high photosynthetic performance of C4 grasses. In Proceedings of the 99th ESA Annual Convention, Sacramento, CA, USA, 10–15 August 2014. [Google Scholar]





| Species and Treatment | Above-Ground Biomass (g) | Below-Ground Biomass (g) | Total Biomass (g) | Root: Shoot Ratio |
|---|---|---|---|---|
| P. centrasiaticum, well-watered | 21.86 ± 3.07 Aa | 35.72 ± 2.54 Aa | 57.58 ± 5.11 Aa | 1.69 ± 0.21 Aa |
| P. centrasiaticum, drought-stressed | 19.45 ± 3.28 Aa | 16.74 ± 1.24 Ba | 36.19 ± 2.08 Ba | 0.93 ± 0.19 Bb |
| C. pseudophragmites, well-watered | 18.73 ± 1.09 Aa | 17.05 ± 2.87 Ab | 35.78 ± 2.99 Ab | 0.92 ± 0.17 Bb |
| C. pseudophragmites, drought-stressed | 5.07 ± 0.48 Bb | 7.12 ± 0.47 Bb | 12.19 ± 0.60 Bb | 1.44 ± 0.20 Aa |
| Species and Treatment | Pn, Biomass (Above-Ground, Below-ground and Total) | WUE | Root: Shoot Ratio | DI0/CS0 | gs, FV/FM and ET0/CS0 | Ci |
|---|---|---|---|---|---|---|
| P. centrasiaticum, well-watered | high | high | high | high | low | low |
| P. centrasiaticum, drought-stressed | reduce less | reduce | reduce | increase | reduce | increase more |
| C. pseudophragmites, well-watered | low | low | low | low | high | high |
| C. pseudophragmites, drought-stressed | reduce more | reduce | increase | increase | reduce | reduce less |
| Terms and Formulae | Explanations |
|---|---|
| FV/FM = (TR0/RC)/(ABS/RC) = TR0/ABS = [1–(F0/FM)] | Maximum quantum yield for PS II |
| ET0/CS0= (ET0/RC)/(ABS/RC)·(ABS/CS0) = (ET0/ABS)·(ABS/CS0) | Electron transport flux per CS |
| DI0/CS0= (ABS/CS0)–(TR0/CS0) | Dissipated energy flux per CS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Zhao, X.; Allington, G.R.H.; Wang, L.; Huang, W.; Zhang, R.; Luo, Y.; Xu, Z. Photosynthesis and Growth of Pennisetum centrasiaticum (C4) is Superior to Calamagrostis pseudophragmites (C3) during Drought and Recovery. Plants 2020, 9, 991. https://doi.org/10.3390/plants9080991
Luo Y, Zhao X, Allington GRH, Wang L, Huang W, Zhang R, Luo Y, Xu Z. Photosynthesis and Growth of Pennisetum centrasiaticum (C4) is Superior to Calamagrostis pseudophragmites (C3) during Drought and Recovery. Plants. 2020; 9(8):991. https://doi.org/10.3390/plants9080991
Chicago/Turabian StyleLuo, Yayong, Xueyong Zhao, Ginger R. H. Allington, Lilong Wang, Wenda Huang, Rui Zhang, Yongqing Luo, and Zhuwen Xu. 2020. "Photosynthesis and Growth of Pennisetum centrasiaticum (C4) is Superior to Calamagrostis pseudophragmites (C3) during Drought and Recovery" Plants 9, no. 8: 991. https://doi.org/10.3390/plants9080991