Oncogene ( IF 8 ) Pub Date : 2019-01-29 , DOI: 10.1038/s41388-018-0650-0 Erik S. Knudsen , Vishnu Kumarasamy , Amanda Ruiz , Jared Sivinski , Sejin Chung , Adam Grant , Paris Vail , Shailender S. Chauhan , Tun Jie , Taylor S. Riall , Agnieszka K. Witkiewicz
|
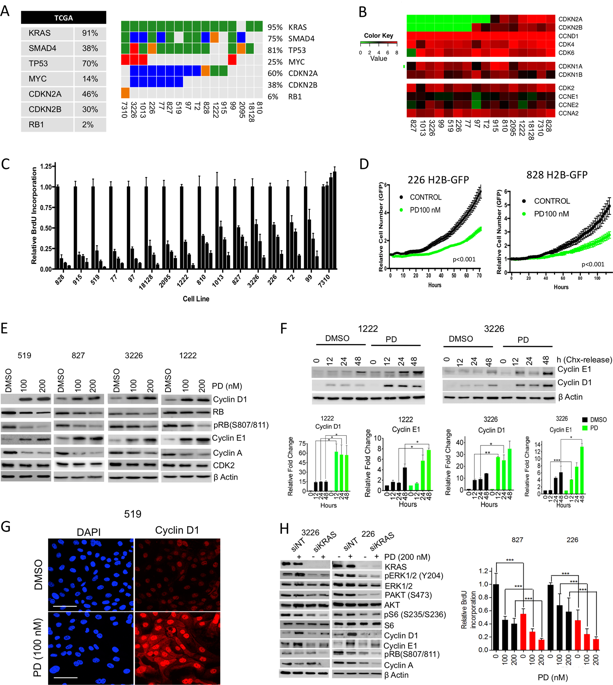
Pancreatic ductal adenocarcinoma (PDAC), like many KRAS-driven tumors, preferentially loses CDKN2A that encodes an endogenous CDK4/6 inhibitor to bypass the RB-mediated cell cycle suppression. Analysis of a panel of patient-derived cell lines and matched xenografts indicated that many pancreatic cancers have intrinsic resistance to CDK4/6 inhibition that is not due to any established mechanism or published biomarker. Rather, there is a KRAS-dependent rapid adaptive response that leads to the upregulation of cyclin proteins, which participate in functional complexes to mediate resistance. In vivo, the degree of response is associated with the suppression of a gene expression signature that is strongly prognostic in pancreatic cancer. Resistance is associated with an adaptive gene expression signature that is common to multiple kinase inhibitors, but is attenuated with MTOR inhibitors. Combination treatment with MTOR and CDK4/6 inhibitors had potent activity across a large number of patient-derived models of PDAC underscoring the potential clinical efficacy.
中文翻译:

MTOR信号驱动的细胞周期可塑性:胰腺癌患者衍生模型中对CDK4 / 6抑制的整体抗性
像许多KRAS驱动的肿瘤一样,胰腺导管腺癌(PDAC)优先丢失编码内源性CDK4 / 6抑制剂的CDKN2A,从而绕过RB介导的细胞周期抑制。对一组患者来源的细胞系和匹配的异种移植物的分析表明,许多胰腺癌具有对CDK4 / 6抑制的内在抗性,这不是由于任何已建立的机制或已发表的生物标记所致。相反,存在依赖于KRAS的快速适应性反应,从而导致细胞周期蛋白的上调,而细胞周期蛋白参与功能性复合物以介导抗性。在体内,反应程度与胰腺癌预后强烈的基因表达特征的抑制有关。抗性与多种激酶抑制剂常见的适应性基因表达特征相关,但是会被MTOR抑制剂减弱。MTOR和CDK4 / 6抑制剂的联合治疗在众多患者衍生的PDAC模型中均具有强大的活性,从而强调了潜在的临床疗效。


























 京公网安备 11010802027423号
京公网安备 11010802027423号