Oncogene ( IF 8 ) Pub Date : 2019-01-28 , DOI: 10.1038/s41388-019-0701-1 Ping Lu , Jing Geng , Lei Zhang , Yu Wang , Ningning Niu , Yuan Fang , Fang Liu , Juanjuan Shi , Zhi-Gang Zhang , Yong-Wei Sun , Li-Wei Wang , Yujie Tang , Jing Xue
|
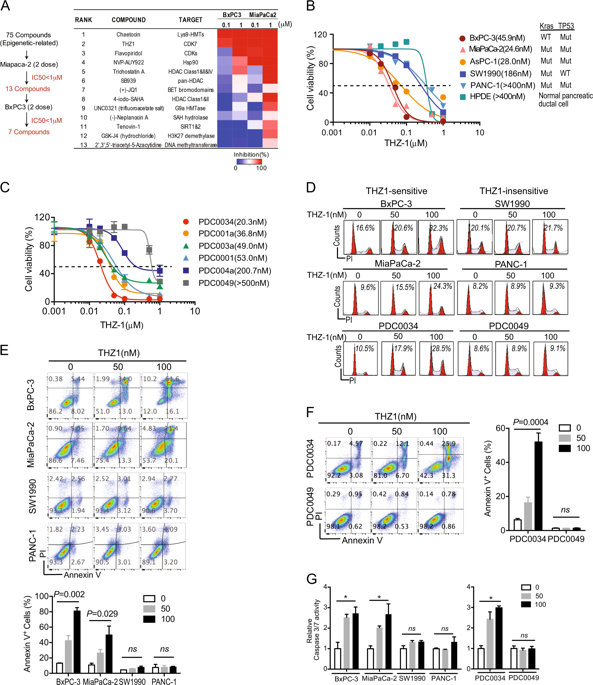
Pancreatic ductal adenocarcinoma (PDAC) is a lethal malignancy with high mortality. Lack of effective treatment makes novel therapeutic discovery an urgent demand in PDAC research. By screening an epigenetic-related compound library, we identified THZ1, a covalent inhibitor of CDK7, as a promising candidate. Multiple long-established and patient-derived PDAC cell lines (PDC) were used to validate the efficacy of THZ1 in vitro. In addition, patient-derived xenograft (PDX) models and animal models of PDAC were utilized for examining THZ1 efficacy in vivo. Furthermore, RNA-Seq analyse was performed to reveal the molecular mechanism of THZ1 treatment. Finally, PDAC cell lines with primary or acquired resistance to THZ1 were investigated to explore the potential mechanism of THZ1 susceptibility. CDK7 inhibition was identified as a selective and potent therapeutic strategy for PDAC progression in multiple preclinical models. Mechanistic analyses revealed that CDK7 inhibition led to a pronounced downregulation of gene transcription, with a preferential repression of mitotic cell cycle and NF-κB signaling-related transcripts. MYC transcriptional was found to be involved in susceptibility of PDAC cells to CDK7 inhibition. In conclusion, Identification of CDK7-dependent transcriptional addiction in PDACs provides a potent therapeutic strategy that targets highly aggressive pancreatic cancer.
中文翻译:

THZ1揭示了胰腺癌中CDK7依赖的转录成瘾
胰腺导管腺癌(PDAC)是一种致命的恶性肿瘤,死亡率高。有效治疗的缺乏使新颖的治疗发现成为PDAC研究的迫切需求。通过筛选表观遗传学相关的化合物库,我们确定了CDZ7的共价抑制剂THZ1是有前途的候选人。多个长期建立且源自患者的PDAC细胞系(PDC)用于在体外验证THZ1的功效。此外,患者来源的异种移植(PDX)模型和PDAC的动物模型用于检查体内THZ1的功效。此外,进行了RNA-Seq分析以揭示THZ1处理的分子机制。最后,对原发性或获得性抗THZ1的PDAC细胞系进行了研究,以探索THZ1敏感性的潜在机制。CDK7抑制被确定为多种临床前模型中PDAC进展的选择性和有效治疗策略。机理分析表明,CDK7抑制导致基因转录的明显下调,并优先抑制有丝分裂细胞周期和NF-κB信号相关的转录本。发现MYC转录与PDAC细胞对CDK7抑制的敏感性有关。总之,在PDAC中CDK7依赖性转录成瘾的鉴定提供了针对高侵袭性胰腺癌的有效治疗策略。优先抑制有丝分裂细胞周期和NF-κB信号相关的转录本。发现MYC转录与PDAC细胞对CDK7抑制的敏感性有关。总之,在PDAC中CDK7依赖性转录成瘾的鉴定提供了针对高侵袭性胰腺癌的有效治疗策略。优先抑制有丝分裂细胞周期和NF-κB信号相关的转录本。发现MYC转录与PDAC细胞对CDK7抑制的敏感性有关。总之,在PDAC中CDK7依赖性转录成瘾的鉴定提供了针对高侵袭性胰腺癌的有效治疗策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号