当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoredox‐Catalyzed Cyclobutane Synthesis by a Deboronative Radical Addition–Polar Cyclization Cascade
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2019-02-15 , DOI: 10.1002/anie.201813917 Chao Shu 1 , Adam Noble 1 , Varinder K Aggarwal 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2019-02-15 , DOI: 10.1002/anie.201813917 Chao Shu 1 , Adam Noble 1 , Varinder K Aggarwal 1
Affiliation
|
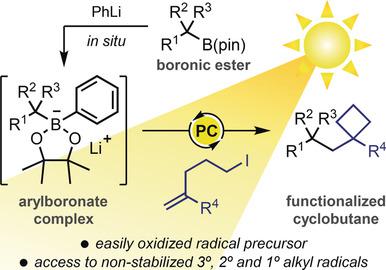
Photoredox‐catalyzed methylcyclobutanations of alkylboronic esters are described. The reactions proceed through single‐electron transfer induced deboronative radical addition to an electron‐deficient alkene followed by single‐electron reduction and polar 4‐exo‐tet cyclization with a pendant alkyl halide. Key to the success of the methodology was the use of easily oxidizable arylboronate complexes. Structurally diverse cyclobutanes are shown to be conveniently prepared from readily available alkylboronic esters and a range of haloalkyl alkenes. The mild reactions display excellent functional group tolerance, and the radical addition‐polar cyclization cascade also enables the synthesis of 3‐, 5‐, 6‐, and 7‐membered rings.
中文翻译:

脱硼自由基加成-极性环化级联光氧化还原催化环丁烷合成
描述了烷基硼酸酯的光氧化还原催化的甲基环丁烷化。该反应通过单电子转移诱导的脱硼自由基加成到缺电子烯烃上,然后进行单电子还原和极性 4- exo - tet环化与侧烷基卤化物。该方法成功的关键是使用易于氧化的芳基硼酸配合物。结构多样的环丁烷可以方便地由容易获得的烷基硼酸酯和一系列卤代烷基烯烃制备。温和的反应表现出优异的官能团耐受性,自由基加成-极性环化级联也能够合成3-、5-、6-和7-元环。
更新日期:2019-02-15
中文翻译:

脱硼自由基加成-极性环化级联光氧化还原催化环丁烷合成
描述了烷基硼酸酯的光氧化还原催化的甲基环丁烷化。该反应通过单电子转移诱导的脱硼自由基加成到缺电子烯烃上,然后进行单电子还原和极性 4- exo - tet环化与侧烷基卤化物。该方法成功的关键是使用易于氧化的芳基硼酸配合物。结构多样的环丁烷可以方便地由容易获得的烷基硼酸酯和一系列卤代烷基烯烃制备。温和的反应表现出优异的官能团耐受性,自由基加成-极性环化级联也能够合成3-、5-、6-和7-元环。



























 京公网安备 11010802027423号
京公网安备 11010802027423号