npj Breast Cancer ( IF 5.9 ) Pub Date : 2019-01-17 , DOI: 10.1038/s41523-018-0097-z Stephen Johnston 1 , Miguel Martin 2 , Angelo Di Leo 3 , Seock-Ah Im 4 , Ahmad Awada 5 , Tammy Forrester 6 , Martin Frenzel 6 , Molly C Hardebeck 7 , Joanne Cox 8 , Susana Barriga 9 , Masakazu Toi 10 , Hiroji Iwata 11 , Matthew P Goetz 12
|
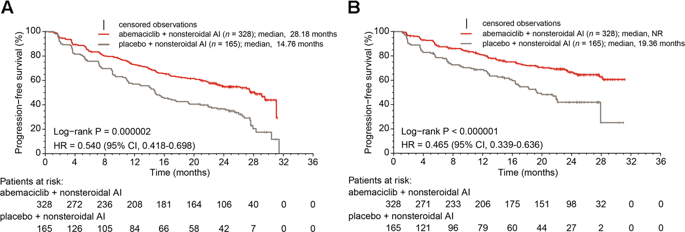
At the MONARCH 3 interim analysis, abemaciclib plus a nonsteroidal aromatase inhibitor (AI) significantly improved progression-free survival (PFS) and objective response rate (ORR) with a tolerable safety profile as initial treatment for hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (ABC). MONARCH 3 is a randomized, phase III, double-blind study of abemaciclib/placebo (150 mg twice daily, continuous) plus nonsteroidal AI (1 mg anastrozole or 2.5 mg letrozole, daily). A total of 493 postmenopausal women with HR+, HER2− ABC with no prior systemic therapy in this setting were enrolled. The primary endpoint was investigator-assessed PFS (final analysis after 240 events); other endpoints included response and safety evaluations. Here we analyze the final PFS data and update secondary endpoints. The abemaciclib arm had a significantly longer median PFS than the placebo arm (28.18 versus 14.76 months; hazard ratio [95% confidence interval], 0.540 [0.418–0.698]; p = .000002). The ORR was 61.0% in the abemaciclib arm versus 45.5% in the placebo arm (measurable disease, p = .003). The median duration of response was longer in the abemaciclib arm (27.39 months) compared to the placebo arm (17.46 months). The safety profile was consistent with previous reports. The most frequent grade ≥ 3 adverse events in the abemaciclib versus placebo arms were neutropenia (23.9% versus 1.2%), diarrhea (9.5% versus 1.2%), and leukopenia (8.6% versus 0.6%). Abemaciclib plus a nonsteroidal AI was an effective initial treatment with an acceptable safety profile for HR+, HER2− ABC.
中文翻译:

MONARCH 3 最终 PFS:abemaciclib 作为晚期乳腺癌初始治疗的随机研究
在 MONARCH 3 中期分析中,abemaciclib 联合非甾体芳香酶抑制剂 (AI) 显着改善了无进展生存期 (PFS) 和客观缓解率 (ORR),并且作为激素受体阳性 (HR+)、人类的初始治疗具有可容忍的安全性。表皮生长因子受体 2 阴性 (HER2−) 晚期乳腺癌 (ABC)。MONARCH 3 是一项随机、III 期、双盲研究,研究对象为 abemaciclib/安慰剂(150 毫克,每日两次,连续)加非类固醇 AI(1 毫克阿那曲唑或 2.5 毫克来曲唑,每日)。共有 493 名患有 HR+、HER2− ABC 且之前未接受全身治疗的绝经后女性入组。主要终点是研究者评估的 PFS(240 个事件后的最终分析);其他终点包括反应和安全性评估。在这里,我们分析最终的 PFS 数据并更新次要终点。abemaciclib 组的中位 PFS 明显长于安慰剂组(28.18 个月与 14.76 个月;风险比 [95% 置信区间],0.540 [0.418–0.698];p = .000002)。abemaciclib 组的 ORR 为 61.0%,而安慰剂组为 45.5%(可测量疾病,p = .003)。与安慰剂组(17.46 个月)相比,abemaciclib 组的中位缓解持续时间(27.39 个月)更长。安全性与之前的报告一致。abemaciclib 组与安慰剂组相比,最常见的 3 级不良事件是中性粒细胞减少症(23.9% 对 1.2%)、腹泻(9.5% 对 1.2%)和白细胞减少症(8.6% 对 0.6%)。Abemaciclib 加非类固醇 AI 是一种有效的初始治疗,对于 HR+、HER2− ABC 具有可接受的安全性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号