PLOS ONE ( IF 3.7 ) Pub Date : 2019-01-15 , DOI: 10.1371/journal.pone.0210833 Anne E Wormsbecker 1, 2, 3 , Caitlin Johnson 4 , Laura Bourns 5 , Tara Harris 4 , Natasha S Crowcroft 4, 6, 7 , Shelley L Deeks 4, 7
|
Introduction
Adverse events following immunization (AEFIs) are unwanted or unexpected health outcomes following vaccination, which may or may not be causally-linked to vaccines. AEFI reporting is important to post-marketing vaccine safety surveillance and has the potential to identify new or rare AEFIs, show increases in known AEFIs, and help to maintain public confidence in vaccine programs. Knowledge of the expected incidence (i.e. background rate) of a possible AEFI is essential to the investigation of vaccine safety signals. We selected three rarely reported AEFIs representing the spectrum of causal association with vaccines, from proven (immune thrombocytopenia [ITP]) to questioned (Kawasaki disease [KD]) to unsubstantiated (multiple sclerosis [MS]) and determined their background rates.
Methods
We extracted data on hospitalizations (CIHI Discharge Abstract Database) for ITP, KD, and MS among Ontario children for the period 2005 to 2014 from IntelliHEALTH. As ITP can be managed without hospitalization, we also extracted emergency department (ED) visits from the CIHI National Ambulatory Care Reporting System. For all conditions, we only counted the first visit and if the same child had both an ED visit and a hospitalization for ITP, only the hospitalization was included. We calculated rates by year, age group and sex using population estimates from 2005–2014, focusing on age groups within the Ontario immunization schedule around vaccine(s) of interest.
Results
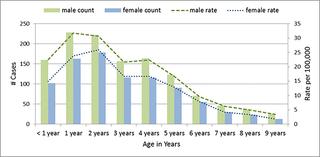
Per 100,000 population, annual age-specific incidence of ITP in children age 1 to 7 years ranged from 8.9 to 12.2 and annual incidence of KD in children less than 5 years ranged from 19.1 to 32.1. Average annualized incidence of adolescent (11–17 years) MS across the study period was 0.8 per 100,000.
Discussion
Despite limitations, including lack of clinical validation, this study provides an example of how health administrative data can be used to determine background rates which may assist with interpretation of passive vaccine safety surveillance.
中文翻译:

疫苗安全监测三个感兴趣条件的背景率展示
介绍
免疫接种后的不良事件 (AEFI) 是接种疫苗后出现的不良或意外的健康结果,可能与疫苗有因果关系,也可能没有因果关系。AEFI 报告对于上市后疫苗安全监测非常重要,并且有可能识别新的或罕见的 AEFI,显示已知 AEFI 的增加,并有助于维持公众对疫苗计划的信心。了解可能的 AEFI 的预期发生率(即背景率)对于疫苗安全信号的调查至关重要。我们选择了三种罕见报告的 AEFI,代表了与疫苗因果关系的范围,从已证实的(免疫性血小板减少症 [ITP])到可疑的(川崎病 [KD])到未经证实的(多发性硬化症 [MS]),并确定了它们的背景率。
方法
我们从 IntelliHEALTH 中提取了 2005 年至 2014 年期间安大略省儿童 ITP、KD 和 MS 的住院数据(CIHI 出院摘要数据库)。由于 ITP 无需住院即可治疗,我们还从 CIHI 国家门诊护理报告系统中提取了急诊室 (ED) 就诊信息。对于所有情况,我们只计算第一次就诊,如果同一个孩子同时因 ITP 就诊和住院,则仅包括住院治疗。我们使用 2005 年至 2014 年的人口估计值按年份、年龄组和性别计算了比率,重点关注安大略省免疫接种计划内感兴趣的疫苗的年龄组。
结果
每10万人中,1至7岁儿童ITP的年发病率为8.9至12.2,5岁以下儿童的KD年发病率为19.1至32.1。研究期间青少年(11-17 岁)MS 的平均年发病率为每 100,000 人 0.8 例。
讨论
尽管存在局限性,包括缺乏临床验证,但本研究提供了一个示例,说明如何使用卫生管理数据来确定背景率,这可能有助于解释被动疫苗安全监测。


























 京公网安备 11010802027423号
京公网安备 11010802027423号