Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Microbiotas from Humans with Inflammatory Bowel Disease Alter the Balance of Gut Th17 and RORγt+ Regulatory T Cells and Exacerbate Colitis in Mice.
Immunity ( IF 32.4 ) Pub Date : 2019-01-15 , DOI: 10.1016/j.immuni.2018.12.015 Graham J Britton 1 , Eduardo J Contijoch 1 , Ilaria Mogno 1 , Olivia H Vennaro 1 , Sean R Llewellyn 1 , Ruby Ng 1 , Zhihua Li 1 , Arthur Mortha 2 , Miriam Merad 3 , Anuk Das 4 , Dirk Gevers 4 , Dermot P B McGovern 5 , Namita Singh 6 , Jonathan Braun 7 , Jonathan P Jacobs 8 , Jose C Clemente 1 , Ari Grinspan 9 , Bruce E Sands 9 , Jean-Frederic Colombel 9 , Marla C Dubinsky 10 , Jeremiah J Faith 1
Immunity ( IF 32.4 ) Pub Date : 2019-01-15 , DOI: 10.1016/j.immuni.2018.12.015 Graham J Britton 1 , Eduardo J Contijoch 1 , Ilaria Mogno 1 , Olivia H Vennaro 1 , Sean R Llewellyn 1 , Ruby Ng 1 , Zhihua Li 1 , Arthur Mortha 2 , Miriam Merad 3 , Anuk Das 4 , Dirk Gevers 4 , Dermot P B McGovern 5 , Namita Singh 6 , Jonathan Braun 7 , Jonathan P Jacobs 8 , Jose C Clemente 1 , Ari Grinspan 9 , Bruce E Sands 9 , Jean-Frederic Colombel 9 , Marla C Dubinsky 10 , Jeremiah J Faith 1
Affiliation
|
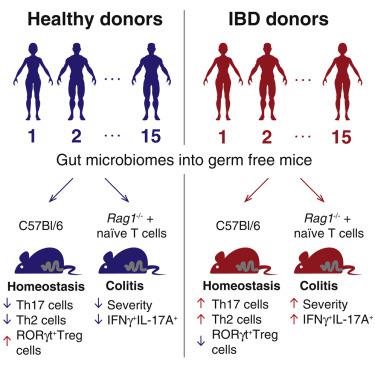
Microbiota are thought to influence the development and progression of inflammatory bowel disease (IBD), but determining generalizable effects of microbiota on IBD etiology requires larger-scale functional analyses. We colonized germ-free mice with intestinal microbiotas from 30 healthy and IBD donors and determined the homeostatic intestinal T cell response to each microbiota. Compared to microbiotas from healthy donors, transfer of IBD microbiotas into germ-free mice increased numbers of intestinal Th17 cells and Th2 cells and decreased numbers of RORγt+ Treg cells. Colonization with IBD microbiotas exacerbated disease in a model where colitis is induced upon transfer of naive T cells into Rag1-/- mice. The proportions of Th17 and RORγt+ Treg cells induced by each microbiota were predictive of human disease status and accounted for disease severity in the Rag1-/- colitis model. Thus, an impact on intestinal Th17 and RORγt+ Treg cell compartments emerges as a unifying feature of IBD microbiotas, suggesting a general mechanism for microbial contribution to IBD pathogenesis.
中文翻译:

来自炎症性肠病患者的微生物群改变肠道 Th17 和 RORγt+ 调节性 T 细胞的平衡并加剧小鼠的结肠炎。
微生物群被认为会影响炎症性肠病 (IBD) 的发展和进展,但确定微生物群对 IBD 病因的普遍影响需要更大规模的功能分析。我们用来自 30 名健康和 IBD 供体的肠道微生物群定殖无菌小鼠,并确定了对每个微生物群的稳态肠道 T 细胞反应。与来自健康供体的微生物群相比,将 IBD 微生物群转移到无菌小鼠体内会增加肠道 Th17 细胞和 Th2 细胞的数量,并减少 RORγt+ Treg 细胞的数量。在将幼稚 T 细胞转移到 Rag1-/- 小鼠后诱导结肠炎的模型中,IBD 微生物群的定植加剧了疾病。每个微生物群诱导的 Th17 和 RORγt+ Treg 细胞的比例可预测人类疾病状态,并解释 Rag1-/- 结肠炎模型中的疾病严重程度。因此,对肠道 Th17 和 RORγt+ Treg 细胞区室的影响成为 IBD 微生物群的统一特征,表明微生物对 IBD 发病机制的贡献的一般机制。
更新日期:2019-01-15
中文翻译:

来自炎症性肠病患者的微生物群改变肠道 Th17 和 RORγt+ 调节性 T 细胞的平衡并加剧小鼠的结肠炎。
微生物群被认为会影响炎症性肠病 (IBD) 的发展和进展,但确定微生物群对 IBD 病因的普遍影响需要更大规模的功能分析。我们用来自 30 名健康和 IBD 供体的肠道微生物群定殖无菌小鼠,并确定了对每个微生物群的稳态肠道 T 细胞反应。与来自健康供体的微生物群相比,将 IBD 微生物群转移到无菌小鼠体内会增加肠道 Th17 细胞和 Th2 细胞的数量,并减少 RORγt+ Treg 细胞的数量。在将幼稚 T 细胞转移到 Rag1-/- 小鼠后诱导结肠炎的模型中,IBD 微生物群的定植加剧了疾病。每个微生物群诱导的 Th17 和 RORγt+ Treg 细胞的比例可预测人类疾病状态,并解释 Rag1-/- 结肠炎模型中的疾病严重程度。因此,对肠道 Th17 和 RORγt+ Treg 细胞区室的影响成为 IBD 微生物群的统一特征,表明微生物对 IBD 发病机制的贡献的一般机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号