当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Two Tryptophans Are Better Than One in Accelerating Electron Flow through a Protein.
ACS Central Science ( IF 18.2 ) Pub Date : 2019-01-07 , DOI: 10.1021/acscentsci.8b00882 Kana Takematsu 1 , Heather R Williamson 2 , Pavle Nikolovski 3 , Jens T Kaiser 3 , Yuling Sheng 3 , Petr Pospíšil 4 , Michael Towrie 5 , Jan Heyda 4, 6 , Daniel Hollas 6 , Stanislav Záliš 4 , Harry B Gray 3 , Antonín Vlček 4, 7 , Jay R Winkler 3
ACS Central Science ( IF 18.2 ) Pub Date : 2019-01-07 , DOI: 10.1021/acscentsci.8b00882 Kana Takematsu 1 , Heather R Williamson 2 , Pavle Nikolovski 3 , Jens T Kaiser 3 , Yuling Sheng 3 , Petr Pospíšil 4 , Michael Towrie 5 , Jan Heyda 4, 6 , Daniel Hollas 6 , Stanislav Záliš 4 , Harry B Gray 3 , Antonín Vlček 4, 7 , Jay R Winkler 3
Affiliation
|
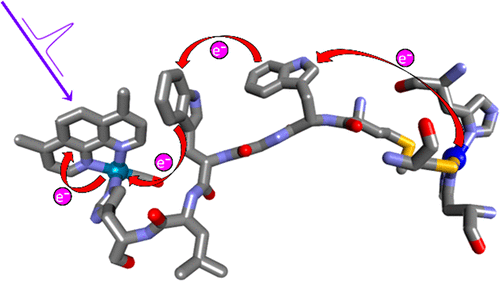
We have constructed and structurally characterized a Pseudomonas aeruginosa azurin mutant Re126WWCuI , where two adjacent tryptophan residues (W124 and W122, indole separation 3.6-4.1 Å) are inserted between the CuI center and a Re photosensitizer coordinated to the imidazole of H126 (ReI(H126)(CO)3(4,7-dimethyl-1,10-phenanthroline)+). CuI oxidation by the photoexcited Re label (*Re) 22.9 Å away proceeds with a ∼70 ns time constant, similar to that of a single-tryptophan mutant (∼40 ns) with a 19.4 Å Re-Cu distance. Time-resolved spectroscopy (luminescence, visible and IR absorption) revealed two rapid reversible electron transfer steps, W124 → *Re (400-475 ps, K 1 ≅ 3.5-4) and W122 → W124•+ (7-9 ns, K 2 ≅ 0.55-0.75), followed by a rate-determining (70-90 ns) CuI oxidation by W122•+ ca. 11 Å away. The photocycle is completed by 120 μs recombination. No photochemical CuI oxidation was observed in Re126FWCuI , whereas in Re126WFCuI , the photocycle is restricted to the ReH126W124 unit and CuI remains isolated. QM/MM/MD simulations of Re126WWCuI indicate that indole solvation changes through the hopping process and W124 → *Re electron transfer is accompanied by water fluctuations that tighten W124 solvation. Our finding that multistep tunneling (hopping) confers a ∼9000-fold advantage over single-step tunneling in the double-tryptophan protein supports the proposal that hole-hopping through tryptophan/tyrosine chains protects enzymes from oxidative damage.
中文翻译:

两种色氨酸比一种色氨酸更能加速电子流过蛋白质。
我们构建了铜绿假单胞菌天青蛋白突变体 Re126WWCuI 并进行结构表征,其中两个相邻的色氨酸残基(W124 和 W122,吲哚间隔 3.6-4.1 Å)插入 CuI 中心和与 H126 的咪唑配位的 Re 光敏剂之间(ReI(H126 )(CO)3(4,7-二甲基-1,10-菲咯啉)+)。光激发 Re 标记 (*Re) 22.9 Å 距离的 CuI 氧化过程的时间常数为 ∼70 ns,与具有 19.4 Å Re-Cu 距离的单色氨酸突变体 (∼40 ns) 相似。时间分辨光谱(发光、可见光和红外吸收)揭示了两个快速可逆电子转移步骤,W124 → *Re (400-475 ps, K 1 ≅ 3.5-4) 和 W122 → W124•+ (7-9 ns, K 2 ≅ 0.55-0.75),然后由 W122•+ 约进行速率决定 (70-90 ns) CuI 氧化。11 Å 远。光循环通过120μs复合完成。在 Re126FWCuI 中没有观察到光化学 CuI 氧化,而在 Re126WFCuI 中,光循环仅限于 ReH126W124 单元并且 CuI 保持孤立状态。Re126WWCuI 的 QM/MM/MD 模拟表明,吲哚溶剂化通过跳跃过程发生变化,并且 W124 → *Re 电子转移伴随着水波动,从而加强了 W124 溶剂化。我们发现,在双色氨酸蛋白中,多步隧道(跳跃)比单步隧道具有约 9000 倍的优势,这支持了这样的观点:通过色氨酸/酪氨酸链的空穴跳跃可以保护酶免受氧化损伤。
更新日期:2019-01-07
中文翻译:

两种色氨酸比一种色氨酸更能加速电子流过蛋白质。
我们构建了铜绿假单胞菌天青蛋白突变体 Re126WWCuI 并进行结构表征,其中两个相邻的色氨酸残基(W124 和 W122,吲哚间隔 3.6-4.1 Å)插入 CuI 中心和与 H126 的咪唑配位的 Re 光敏剂之间(ReI(H126 )(CO)3(4,7-二甲基-1,10-菲咯啉)+)。光激发 Re 标记 (*Re) 22.9 Å 距离的 CuI 氧化过程的时间常数为 ∼70 ns,与具有 19.4 Å Re-Cu 距离的单色氨酸突变体 (∼40 ns) 相似。时间分辨光谱(发光、可见光和红外吸收)揭示了两个快速可逆电子转移步骤,W124 → *Re (400-475 ps, K 1 ≅ 3.5-4) 和 W122 → W124•+ (7-9 ns, K 2 ≅ 0.55-0.75),然后由 W122•+ 约进行速率决定 (70-90 ns) CuI 氧化。11 Å 远。光循环通过120μs复合完成。在 Re126FWCuI 中没有观察到光化学 CuI 氧化,而在 Re126WFCuI 中,光循环仅限于 ReH126W124 单元并且 CuI 保持孤立状态。Re126WWCuI 的 QM/MM/MD 模拟表明,吲哚溶剂化通过跳跃过程发生变化,并且 W124 → *Re 电子转移伴随着水波动,从而加强了 W124 溶剂化。我们发现,在双色氨酸蛋白中,多步隧道(跳跃)比单步隧道具有约 9000 倍的优势,这支持了这样的观点:通过色氨酸/酪氨酸链的空穴跳跃可以保护酶免受氧化损伤。


























 京公网安备 11010802027423号
京公网安备 11010802027423号