当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Functional Proteomics of Breast Cancer Metabolism Identifies GLUL as Responder during Hypoxic Adaptation.
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2019-01-18 , DOI: 10.1021/acs.jproteome.8b00944 Stephan Bernhardt 1 , Christian Tönsing 2 , Devina Mitra 1 , Nese Erdem 1, 3 , Karin Müller-Decker 4 , Ulrike Korf 1 , Clemens Kreutz 5, 6 , Jens Timmer 2, 5, 6 , Stefan Wiemann 1, 3
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2019-01-18 , DOI: 10.1021/acs.jproteome.8b00944 Stephan Bernhardt 1 , Christian Tönsing 2 , Devina Mitra 1 , Nese Erdem 1, 3 , Karin Müller-Decker 4 , Ulrike Korf 1 , Clemens Kreutz 5, 6 , Jens Timmer 2, 5, 6 , Stefan Wiemann 1, 3
Affiliation
|
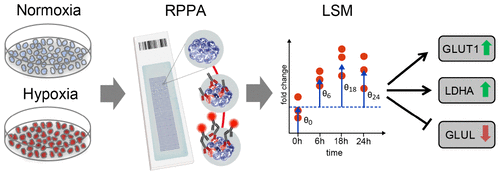
Hypoxia as well as metabolism are central hallmarks of cancer, and hypoxia-inducible factors (HIFs) and metabolic effectors are crucial elements in oxygen-compromised tumor environments. Knowledge of changes in the expression of metabolic proteins in response to HIF function could provide mechanistic insights into adaptation to hypoxic stress, tumorigenesis, and disease progression. We analyzed time-resolved alterations in metabolism-associated protein levels in response to different oxygen potentials across breast cancer cell lines. Effects on the cellular metabolism of both HIF-dependent and -independent processes were analyzed by reverse-phase protein array profiling and a custom statistical model. We revealed a strong induction of glucose transporter 1 (GLUT1) and lactate dehydrogenase A (LDHA) as well as reduced glutamate-ammonia ligase (GLUL) protein levels across all cell lines tested as consistent changes upon hypoxia induction. Low GLUL protein levels were correlated with aggressive molecular subtypes in breast cancer patient data sets and also with hypoxic tumor regions in a xenograft mouse tumor model. Moreover, low GLUL expression was associated with poor survival in breast cancer patients and with high HIF-1α-expressing patient subgroups. Our data reveal time-resolved changes in the regulation of metabolic proteins under oxygen-deprived conditions and elucidate GLUL as a strong responder to HIFs and the hypoxic environment.
中文翻译:

乳腺癌代谢的功能蛋白质组学确定低氧适应过程中GLUL是应答者。
缺氧以及新陈代谢是癌症的主要特征,而缺氧诱导因子(HIF)和代谢效应因子是缺氧的肿瘤环境中的关键要素。响应HIF功能的代谢蛋白表达变化的知识可以为适应低氧应激,肿瘤发生和疾病进展提供机械学见解。我们分析了乳腺癌细胞系中不同氧势对新陈代谢相关蛋白水平的时间分辨变化。通过反相蛋白质阵列分析和自定义统计模型分析了HIF依赖性和非依赖性过程对细胞代谢的影响。我们发现在所有缺氧诱导的细胞系中,葡萄糖转运蛋白1(GLUT1)和乳酸脱氢酶A(LDHA)的强烈诱导作用以及降低的谷氨酸-氨连接酶(GLUL)蛋白水平均呈恒定变化。低GLUL蛋白水平与乳腺癌患者数据集中的侵略性分子亚型以及异种移植小鼠肿瘤模型中的低氧肿瘤区域相关。此外,低GLUL表达与乳腺癌患者的低生存率以及高表达HIF-1α的患者亚组有关。我们的数据揭示了在缺氧条件下代谢蛋白质调控的时间分辨变化,并阐明了GLUL对HIF和低氧环境的强烈反应。低GLUL蛋白水平与乳腺癌患者数据集中的侵略性分子亚型以及异种移植小鼠肿瘤模型中的低氧肿瘤区域相关。此外,低GLUL表达与乳腺癌患者的低生存率以及高表达HIF-1α的患者亚组有关。我们的数据揭示了在缺氧条件下代谢蛋白质调控的时间分辨变化,并阐明了GLUL对HIF和低氧环境的强烈反应。低GLUL蛋白水平与乳腺癌患者数据集中的侵略性分子亚型以及异种移植小鼠肿瘤模型中的低氧肿瘤区域相关。此外,低GLUL表达与乳腺癌患者的低生存率以及高表达HIF-1α的患者亚组有关。我们的数据揭示了在缺氧条件下代谢蛋白质调控的时间分辨变化,并阐明了GLUL对HIF和低氧环境的强烈反应。
更新日期:2019-02-07
中文翻译:

乳腺癌代谢的功能蛋白质组学确定低氧适应过程中GLUL是应答者。
缺氧以及新陈代谢是癌症的主要特征,而缺氧诱导因子(HIF)和代谢效应因子是缺氧的肿瘤环境中的关键要素。响应HIF功能的代谢蛋白表达变化的知识可以为适应低氧应激,肿瘤发生和疾病进展提供机械学见解。我们分析了乳腺癌细胞系中不同氧势对新陈代谢相关蛋白水平的时间分辨变化。通过反相蛋白质阵列分析和自定义统计模型分析了HIF依赖性和非依赖性过程对细胞代谢的影响。我们发现在所有缺氧诱导的细胞系中,葡萄糖转运蛋白1(GLUT1)和乳酸脱氢酶A(LDHA)的强烈诱导作用以及降低的谷氨酸-氨连接酶(GLUL)蛋白水平均呈恒定变化。低GLUL蛋白水平与乳腺癌患者数据集中的侵略性分子亚型以及异种移植小鼠肿瘤模型中的低氧肿瘤区域相关。此外,低GLUL表达与乳腺癌患者的低生存率以及高表达HIF-1α的患者亚组有关。我们的数据揭示了在缺氧条件下代谢蛋白质调控的时间分辨变化,并阐明了GLUL对HIF和低氧环境的强烈反应。低GLUL蛋白水平与乳腺癌患者数据集中的侵略性分子亚型以及异种移植小鼠肿瘤模型中的低氧肿瘤区域相关。此外,低GLUL表达与乳腺癌患者的低生存率以及高表达HIF-1α的患者亚组有关。我们的数据揭示了在缺氧条件下代谢蛋白质调控的时间分辨变化,并阐明了GLUL对HIF和低氧环境的强烈反应。低GLUL蛋白水平与乳腺癌患者数据集中的侵略性分子亚型以及异种移植小鼠肿瘤模型中的低氧肿瘤区域相关。此外,低GLUL表达与乳腺癌患者的低生存率以及高表达HIF-1α的患者亚组有关。我们的数据揭示了在缺氧条件下代谢蛋白质调控的时间分辨变化,并阐明了GLUL对HIF和低氧环境的强烈反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号