当前位置:
X-MOL 学术
›
Genet. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Loss of function of SVBP leads to autosomal recessive intellectual disability, microcephaly, ataxia, and hypotonia.
Genetics in Medicine ( IF 8.8 ) Pub Date : 2019-01-04 , DOI: 10.1038/s41436-018-0415-8 Zafar Iqbal 1, 2 , Hasan Tawamie 3, 4 , Wei Ba 1 , André Reis 3 , Bassam Al Halak 5 , Heinrich Sticht 6 , Steffen Uebe 3 , Nael Nadif Kasri 1 , Sheikh Riazuddin 7, 8 , Hans van Bokhoven 1 , Rami Abou Jamra 3, 4
Genetics in Medicine ( IF 8.8 ) Pub Date : 2019-01-04 , DOI: 10.1038/s41436-018-0415-8 Zafar Iqbal 1, 2 , Hasan Tawamie 3, 4 , Wei Ba 1 , André Reis 3 , Bassam Al Halak 5 , Heinrich Sticht 6 , Steffen Uebe 3 , Nael Nadif Kasri 1 , Sheikh Riazuddin 7, 8 , Hans van Bokhoven 1 , Rami Abou Jamra 3, 4
Affiliation
|
PURPOSE
Identifying and characterizing novel causes of autosomal recessive intellectual disability based on systematic clinical and genetic evaluation, followed by functional experiments.
METHODS
Clinical examinations, genome-wide positional mapping, and sequencing were followed by quantitative polymerase chain reaction and western blot of the protein SVBP and its interaction partners. We then knocked down the gene in rat primary hippocampal neurons and evaluated the consequences on synapses.
RESULTS
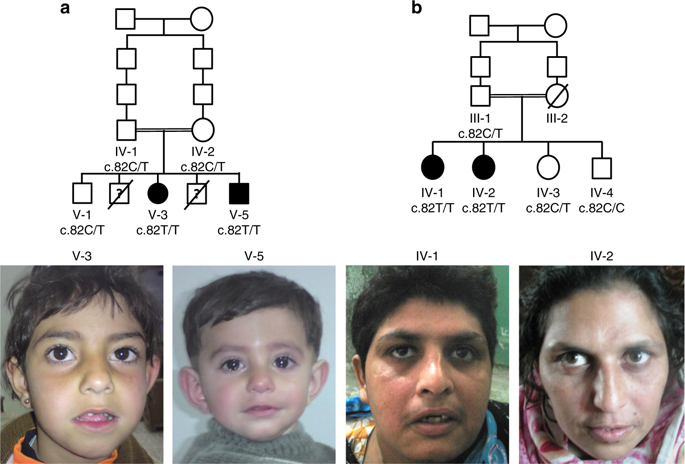
We identified a founder, homozygous stop-gain variant in SVBP (c.82C>T; p.[Gln28*]) in four affected individuals from two independent families with intellectual disability, microcephaly, ataxia, and muscular hypotonia. SVBP encodes a small chaperone protein that transports and stabilizes two angiogenesis regulators, VASH1 and VASH2. The altered protein is unstable and nonfunctional since transfected HeLa cells with mutant SVBP did not reveal evidence for immunoreactive SVBP protein fragments and cotransfection with VASH1 showed a severe reduction of VASH1 in medium and cell lysate. Knocking down Svbp in rat primary hippocampal neurons led to a significant decrease in the number of excitatory synapses.
CONCLUSION
SVBP is not only involved in angiogenesis, but also has vital functions in the central nervous system. Biallelic loss-of-function variants in SVBP lead to intellectual disability.
中文翻译:

SVBP 功能丧失导致常染色体隐性遗传智力残疾、小头畸形、共济失调和肌张力减退。
目的基于系统的临床和遗传评估,然后进行功能实验,识别和表征常染色体隐性智力障碍的新原因。方法临床检查、全基因组位置图谱和测序后,进行定量聚合酶链反应和蛋白质 SVBP 及其相互作用伙伴的蛋白质印迹。然后,我们敲低了大鼠原代海马神经元中的基因,并评估了对突触的影响。结果 我们在来自智力残疾、小头畸形、共济失调和肌张力减退的两个独立家庭的四个受影响个体中发现了 SVBP 中的一个创始人纯合停止增益变异 (c.82C>T;p.[Gln28*])。SVBP 编码一种小型伴侣蛋白,可运输和稳定两种血管生成调节剂 VASH1 和 VASH2。改变的蛋白质不稳定且无功能,因为用突变 SVBP 转染的 HeLa 细胞没有显示免疫反应性 SVBP 蛋白片段的证据,并且与 VASH1 共转染显示培养基和细胞裂解物中 VASH1 的严重减少。敲除大鼠原代海马神经元中的 Svbp 导致兴奋性突触数量显着减少。结论 SVBP不仅参与血管生成,而且在中枢神经系统中具有重要作用。SVBP 中的双等位基因功能丧失变异导致智力障碍。敲除大鼠原代海马神经元中的 Svbp 导致兴奋性突触数量显着减少。结论 SVBP不仅参与血管生成,而且在中枢神经系统中具有重要作用。SVBP 中的双等位基因功能丧失变异导致智力障碍。敲除大鼠原代海马神经元中的 Svbp 导致兴奋性突触数量显着减少。结论 SVBP不仅参与血管生成,而且在中枢神经系统中具有重要作用。SVBP 中的双等位基因功能丧失变异导致智力障碍。
更新日期:2019-01-26
中文翻译:

SVBP 功能丧失导致常染色体隐性遗传智力残疾、小头畸形、共济失调和肌张力减退。
目的基于系统的临床和遗传评估,然后进行功能实验,识别和表征常染色体隐性智力障碍的新原因。方法临床检查、全基因组位置图谱和测序后,进行定量聚合酶链反应和蛋白质 SVBP 及其相互作用伙伴的蛋白质印迹。然后,我们敲低了大鼠原代海马神经元中的基因,并评估了对突触的影响。结果 我们在来自智力残疾、小头畸形、共济失调和肌张力减退的两个独立家庭的四个受影响个体中发现了 SVBP 中的一个创始人纯合停止增益变异 (c.82C>T;p.[Gln28*])。SVBP 编码一种小型伴侣蛋白,可运输和稳定两种血管生成调节剂 VASH1 和 VASH2。改变的蛋白质不稳定且无功能,因为用突变 SVBP 转染的 HeLa 细胞没有显示免疫反应性 SVBP 蛋白片段的证据,并且与 VASH1 共转染显示培养基和细胞裂解物中 VASH1 的严重减少。敲除大鼠原代海马神经元中的 Svbp 导致兴奋性突触数量显着减少。结论 SVBP不仅参与血管生成,而且在中枢神经系统中具有重要作用。SVBP 中的双等位基因功能丧失变异导致智力障碍。敲除大鼠原代海马神经元中的 Svbp 导致兴奋性突触数量显着减少。结论 SVBP不仅参与血管生成,而且在中枢神经系统中具有重要作用。SVBP 中的双等位基因功能丧失变异导致智力障碍。敲除大鼠原代海马神经元中的 Svbp 导致兴奋性突触数量显着减少。结论 SVBP不仅参与血管生成,而且在中枢神经系统中具有重要作用。SVBP 中的双等位基因功能丧失变异导致智力障碍。



























 京公网安备 11010802027423号
京公网安备 11010802027423号