当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Radioimmunotherapy of PANC-1 Human Pancreatic Cancer Xenografts in NRG Mice with Panitumumab Modified with Metal-Chelating Polymers Complexed to 177Lu
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-12-27 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b01040 Sadaf Aghevlian 1 , Zhongli Cai 1 , Yijie Lu 2 , David W. Hedley 3 , Mitchell A. Winnik 2 , Raymond M. Reilly 1, 4, 5
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-12-27 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b01040 Sadaf Aghevlian 1 , Zhongli Cai 1 , Yijie Lu 2 , David W. Hedley 3 , Mitchell A. Winnik 2 , Raymond M. Reilly 1, 4, 5
Affiliation
|
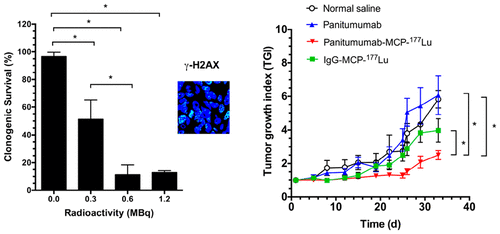
Our aim was to evaluate the effectiveness and normal tissue toxicity of radioimmunotherapy (RIT) of s.c. PANC-1 human pancreatic cancer (PnCa) xenografts in NRG mice using anti-EGFR panitumumab linked to metal-chelating polymers (MCPs) that present 13 DOTA chelators to complex the β-emitter, 177Lu. The clonogenic survival (CS) of PANC-1 cells treated in vitro with panitumumab-MCP-177Lu (0.3–1.2 MBq) and DNA double-strand breaks (DSBs) in the nucleus of these cells were measured by confocal immunofluorescence microscopy for γ-H2AX. Subcellular distribution of radioactivity for panitumumab-MCP-177Lu was measured, and absorbed doses to the cell nucleus were calculated. Normal tissue toxicity was assessed in non tumor-bearing NRG mice by monitoring body weight, complete blood cell counts (CBC), serum alanine aminotransferase (ALT), and creatinine (Cr) after i.v. injection of 6 MBq (10 μg) of panitumumab-MCP-177Lu. RIT was performed in NRG mice with s.c. PANC-1 tumors injected i.v. with 6 MBq (10 μg) of panitumumab-MCP-177Lu. Control mice received nonspecific human IgG-MCP-177Lu (6 MBq; 10 μg), unlabeled panitumumab (10 μg), or normal saline. The tumor growth index (TGI) was compared. Tumor and normal organ doses were estimated based on biodistribution studies. Panitumumab-MCP-177Lu reduced the CS of PANC-1 cells in vitro by 7.7-fold at the highest amount tested (1.2 MBq). Unlabeled panitumumab had no effect on the CS of PANC-1 cells. γ-H2AX foci were increased by 3.8-fold by panitumumab-MCP-177Lu. Panitumumab-MCP-177Lu deposited 3.84 Gy in the nucleus of PANC-1 cells. Administration of panitumumab-MCP-177Lu (6 MBq; 10 μg) to NRG mice caused no change in body weight, CBC, or ALT and only a slight increase in Cr compared to NRG mice treated with normal saline. Panitumumab-MCP-177Lu strongly inhibited tumor growth in NRG mice (TGI = 2.3 ± 0.2) compared to normal saline-treated mice (TGI = 5.8 ± 0.5; P < 0.01). Unlabeled panitumumab had no effect on tumor growth (TGI = 6.0 ± 1.6; P > 0.05). The absorbed dose of PANC-1 tumors was 12.3 Gy. The highest normal organ doses were absorbed by the pancreas, liver, spleen, and kidneys. We conclude that EGFR-targeted RIT with panitumumab-MCP-177Lu was able to overcome resistance to panitumumab in KRAS mutant PANC-1 tumors in NRG mice and may be a promising approach to treatment of PnCa in humans.
中文翻译:

金属螯合聚合物与177 Lu复合修饰的帕尼单抗对NRG小鼠PANC-1人胰腺癌异种移植物的放射免疫治疗。
我们的目的是使用与13种DOTA螯合剂结合的金属螯合聚合物(MCP)的抗EGFR panitumumab评估sc PANC-1人胰腺癌(PnCa)异种移植物在NRG小鼠中的放射免疫疗法(RIT)的有效性和正常组织毒性复配177 Lu的β-发射极 通过共聚焦免疫荧光显微镜检测用panitumumab-MCP- 177 Lu(0.3–1.2 MBq)体外处理的PANC-1细胞的克隆形成存活(CS)和这些细胞核中的DNA双链断裂(DSB)。 -H2AX。帕尼单抗-MCP- 177放射活性的亚细胞分布测量Lu,并计算对细胞核的吸收剂量。通过监测体重,全血细胞计数(CBC),血清丙氨酸氨基转移酶(ALT)和肌酐(Cr)的静脉注射6 MBq(10μg)panitumumab- MCP- 177路。在NRG小鼠中进行了RIT治疗,该小鼠的sc PANC-1肿瘤经静脉注射了6 MBq(10μg)的panitumumab-MCP- 177 Lu。对照小鼠接受非特异性人IgG-MCP- 177 Lu(6 MBq; 10μg),未标记的帕尼单抗(10μg)或生理盐水。比较了肿瘤生长指数(TGI)。根据生物分布研究估算了肿瘤和正常器官的剂量。帕尼单抗-MCP- 177Lu在测试的最高量(1.2 MBq)下将PANC-1细胞的CS降低了7.7倍。未标记的帕尼单抗对PANC-1细胞的CS没有影响。帕尼单抗-MCP- 177 Lu使γ-H2AX病灶增加了3.8倍。Panitumumab-MCP- 177 Lu在PANC-1细胞核中沉积了3.84 Gy。与用生理盐水处理的NRG小鼠相比,向NRG小鼠施用panitumumab-MCP- 177 Lu(6 MBq; 10μg)不会引起体重,CBC或ALT的变化,而Cr只会稍微增加。与生理盐水处理的小鼠(TGI = 5.8±0.5;P <0.01)相比,帕尼单抗-MCP- 177 Lu在NRG小鼠(TGI = 2.3±0.2)中强烈抑制肿瘤生长。未标记的帕尼单抗对肿瘤生长没有影响(TGI = 6.0±1.6;P > 0.05)。PANC-1肿瘤的吸收剂量为12.3 Gy。正常器官最高剂量被胰腺,肝脏,脾脏和肾脏吸收。我们得出的结论是,以panitumumab-MCP- 177 Lu靶向EGFR的RIT能够克服NRG小鼠KRAS突变PANC-1肿瘤对panitumumab的耐药性,可能是治疗人类PnCa的有前途的方法。
更新日期:2018-12-27
中文翻译:

金属螯合聚合物与177 Lu复合修饰的帕尼单抗对NRG小鼠PANC-1人胰腺癌异种移植物的放射免疫治疗。
我们的目的是使用与13种DOTA螯合剂结合的金属螯合聚合物(MCP)的抗EGFR panitumumab评估sc PANC-1人胰腺癌(PnCa)异种移植物在NRG小鼠中的放射免疫疗法(RIT)的有效性和正常组织毒性复配177 Lu的β-发射极 通过共聚焦免疫荧光显微镜检测用panitumumab-MCP- 177 Lu(0.3–1.2 MBq)体外处理的PANC-1细胞的克隆形成存活(CS)和这些细胞核中的DNA双链断裂(DSB)。 -H2AX。帕尼单抗-MCP- 177放射活性的亚细胞分布测量Lu,并计算对细胞核的吸收剂量。通过监测体重,全血细胞计数(CBC),血清丙氨酸氨基转移酶(ALT)和肌酐(Cr)的静脉注射6 MBq(10μg)panitumumab- MCP- 177路。在NRG小鼠中进行了RIT治疗,该小鼠的sc PANC-1肿瘤经静脉注射了6 MBq(10μg)的panitumumab-MCP- 177 Lu。对照小鼠接受非特异性人IgG-MCP- 177 Lu(6 MBq; 10μg),未标记的帕尼单抗(10μg)或生理盐水。比较了肿瘤生长指数(TGI)。根据生物分布研究估算了肿瘤和正常器官的剂量。帕尼单抗-MCP- 177Lu在测试的最高量(1.2 MBq)下将PANC-1细胞的CS降低了7.7倍。未标记的帕尼单抗对PANC-1细胞的CS没有影响。帕尼单抗-MCP- 177 Lu使γ-H2AX病灶增加了3.8倍。Panitumumab-MCP- 177 Lu在PANC-1细胞核中沉积了3.84 Gy。与用生理盐水处理的NRG小鼠相比,向NRG小鼠施用panitumumab-MCP- 177 Lu(6 MBq; 10μg)不会引起体重,CBC或ALT的变化,而Cr只会稍微增加。与生理盐水处理的小鼠(TGI = 5.8±0.5;P <0.01)相比,帕尼单抗-MCP- 177 Lu在NRG小鼠(TGI = 2.3±0.2)中强烈抑制肿瘤生长。未标记的帕尼单抗对肿瘤生长没有影响(TGI = 6.0±1.6;P > 0.05)。PANC-1肿瘤的吸收剂量为12.3 Gy。正常器官最高剂量被胰腺,肝脏,脾脏和肾脏吸收。我们得出的结论是,以panitumumab-MCP- 177 Lu靶向EGFR的RIT能够克服NRG小鼠KRAS突变PANC-1肿瘤对panitumumab的耐药性,可能是治疗人类PnCa的有前途的方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号