当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Label-Free Proteomic Analysis of Exosomes Secreted from THP-1-Derived Macrophages Treated with IFN-α Identifies Antiviral Proteins Enriched in Exosomes.
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2019-01-03 , DOI: 10.1021/acs.jproteome.8b00514 Zhenlan Yao 1 , Xiaofang Jia 2 , Dominik A Megger 3, 4 , Jieliang Chen 1 , Yuyi Liu 1 , Jianhua Li 1 , Barbara Sitek 3 , Zhenghong Yuan 1
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2019-01-03 , DOI: 10.1021/acs.jproteome.8b00514 Zhenlan Yao 1 , Xiaofang Jia 2 , Dominik A Megger 3, 4 , Jieliang Chen 1 , Yuyi Liu 1 , Jianhua Li 1 , Barbara Sitek 3 , Zhenghong Yuan 1
Affiliation
|
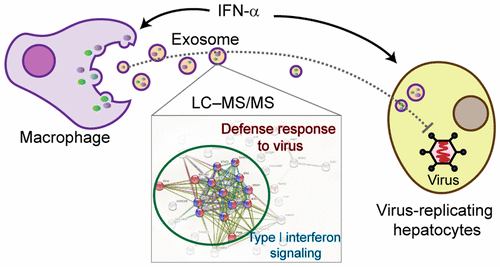
Exosomes are extracellular vesicles that function in intercellular communication. We have previously reported that exosomes play an important role in the transmission of antiviral molecules during interferon-α (IFN-α) treatment. In this study, the protein profiles of THP-1-derived macrophages with or without interferon-α treatment and the exosomes secreted from these cells were analyzed by label-free liquid chromatography-tandem mass spectrometry quantitation technologies. A total of 1845 and 1550 protein groups were identified in the THP-1 macrophages and the corresponding exosomes, respectively. Treating the cells with IFN-α resulted in the differential abundance of 94 proteins in cells and 67 proteins in exosomes (greater than 2.0-fold), among which 23 proteins were up-regulated in both the IFN-α treated cells and corresponding exosomes, while 14 proteins were specifically up-regulated in exosomes but not in the donor cells. GO and KEGG analysis of the identified proteins suggested that IFN-α promoted the abundance of proteins involved in the "defense response to virus" and "type I interferon signaling pathway" in both exosomes and cells. Functional analysis further indicated that exosomes from IFN-α-treated cells exhibited potent antiviral activity that restored the impaired antiviral response of IFN-α in hepatitis B virus-replicating hepatocytes. These results have deepened the understanding of the exosome-mediated transfer of IFN-α-induced antiviral molecules and may provide a new basis for therapeutic strategies to control viral infection.
中文翻译:

用IFN-α处理的THP-1衍生巨噬细胞分泌的外来体的无标签蛋白质组学分析可鉴定出富含外来体的抗病毒蛋白。
外泌体是在细胞间通讯中起作用的细胞外囊泡。我们以前曾报道过,外来体在干扰素-α(IFN-α)治疗期间在抗病毒分子的传播中起着重要作用。在这项研究中,通过无标记液相色谱-串联质谱定量技术分析了有或没有干扰素-α处理的THP-1衍生巨噬细胞的蛋白质谱以及这些细胞分泌的外泌体。在THP-1巨噬细胞和相应的外来体中分别鉴定出总共1845和1550个蛋白质组。用IFN-α处理细胞会导致细胞中94种蛋白质和外泌体中67种蛋白质的差异丰富(大于2.0倍),其中IFN-α处理的细胞和相应的外泌体中有23种蛋白质上调,而14种蛋白质在外来体中特异性上调,而在供体细胞中则没有。对已鉴定蛋白质的GO和KEGG分析表明,IFN-α促进了外泌体和细胞中涉及“病毒防御反应”和“ I型干扰素信号传导途径”的大量蛋白质。功能分析进一步表明,来自IFN-α处理的细胞的外泌体表现出强大的抗病毒活性,可以恢复IFN-α在复制乙型肝炎病毒的肝细胞中受损的抗病毒应答。这些结果加深了对外来体介导的IFN-α诱导的抗病毒分子转移的理解,并可能为控制病毒感染的治疗策略提供新的依据。对已鉴定蛋白质的GO和KEGG分析表明,IFN-α促进了外泌体和细胞中涉及“病毒防御反应”和“ I型干扰素信号传导途径”的大量蛋白质。功能分析进一步表明,来自IFN-α处理的细胞的外泌体表现出强大的抗病毒活性,可以恢复IFN-α在复制乙型肝炎病毒的肝细胞中受损的抗病毒应答。这些结果加深了对外来体介导的IFN-α诱导的抗病毒分子转移的理解,并可能为控制病毒感染的治疗策略提供新的依据。对已鉴定蛋白质的GO和KEGG分析表明,IFN-α促进了外泌体和细胞中涉及“病毒防御反应”和“ I型干扰素信号传导途径”的大量蛋白质。功能分析进一步表明,来自IFN-α处理的细胞的外泌体表现出强大的抗病毒活性,可以恢复IFN-α在复制乙型肝炎病毒的肝细胞中受损的抗病毒应答。这些结果加深了对外来体介导的IFN-α诱导的抗病毒分子转移的理解,并可能为控制病毒感染的治疗策略提供新的依据。功能分析进一步表明,来自IFN-α处理的细胞的外泌体表现出强大的抗病毒活性,可以恢复IFN-α在复制乙型肝炎病毒的肝细胞中受损的抗病毒应答。这些结果加深了对外来体介导的IFN-α诱导的抗病毒分子转移的理解,并可能为控制病毒感染的治疗策略提供新的基础。功能分析进一步表明,来自IFN-α处理的细胞的外泌体表现出强大的抗病毒活性,可以恢复IFN-α在复制乙型肝炎病毒的肝细胞中受损的抗病毒应答。这些结果加深了对外来体介导的IFN-α诱导的抗病毒分子转移的理解,并可能为控制病毒感染的治疗策略提供新的依据。
更新日期:2019-02-07
中文翻译:

用IFN-α处理的THP-1衍生巨噬细胞分泌的外来体的无标签蛋白质组学分析可鉴定出富含外来体的抗病毒蛋白。
外泌体是在细胞间通讯中起作用的细胞外囊泡。我们以前曾报道过,外来体在干扰素-α(IFN-α)治疗期间在抗病毒分子的传播中起着重要作用。在这项研究中,通过无标记液相色谱-串联质谱定量技术分析了有或没有干扰素-α处理的THP-1衍生巨噬细胞的蛋白质谱以及这些细胞分泌的外泌体。在THP-1巨噬细胞和相应的外来体中分别鉴定出总共1845和1550个蛋白质组。用IFN-α处理细胞会导致细胞中94种蛋白质和外泌体中67种蛋白质的差异丰富(大于2.0倍),其中IFN-α处理的细胞和相应的外泌体中有23种蛋白质上调,而14种蛋白质在外来体中特异性上调,而在供体细胞中则没有。对已鉴定蛋白质的GO和KEGG分析表明,IFN-α促进了外泌体和细胞中涉及“病毒防御反应”和“ I型干扰素信号传导途径”的大量蛋白质。功能分析进一步表明,来自IFN-α处理的细胞的外泌体表现出强大的抗病毒活性,可以恢复IFN-α在复制乙型肝炎病毒的肝细胞中受损的抗病毒应答。这些结果加深了对外来体介导的IFN-α诱导的抗病毒分子转移的理解,并可能为控制病毒感染的治疗策略提供新的依据。对已鉴定蛋白质的GO和KEGG分析表明,IFN-α促进了外泌体和细胞中涉及“病毒防御反应”和“ I型干扰素信号传导途径”的大量蛋白质。功能分析进一步表明,来自IFN-α处理的细胞的外泌体表现出强大的抗病毒活性,可以恢复IFN-α在复制乙型肝炎病毒的肝细胞中受损的抗病毒应答。这些结果加深了对外来体介导的IFN-α诱导的抗病毒分子转移的理解,并可能为控制病毒感染的治疗策略提供新的依据。对已鉴定蛋白质的GO和KEGG分析表明,IFN-α促进了外泌体和细胞中涉及“病毒防御反应”和“ I型干扰素信号传导途径”的大量蛋白质。功能分析进一步表明,来自IFN-α处理的细胞的外泌体表现出强大的抗病毒活性,可以恢复IFN-α在复制乙型肝炎病毒的肝细胞中受损的抗病毒应答。这些结果加深了对外来体介导的IFN-α诱导的抗病毒分子转移的理解,并可能为控制病毒感染的治疗策略提供新的依据。功能分析进一步表明,来自IFN-α处理的细胞的外泌体表现出强大的抗病毒活性,可以恢复IFN-α在复制乙型肝炎病毒的肝细胞中受损的抗病毒应答。这些结果加深了对外来体介导的IFN-α诱导的抗病毒分子转移的理解,并可能为控制病毒感染的治疗策略提供新的基础。功能分析进一步表明,来自IFN-α处理的细胞的外泌体表现出强大的抗病毒活性,可以恢复IFN-α在复制乙型肝炎病毒的肝细胞中受损的抗病毒应答。这些结果加深了对外来体介导的IFN-α诱导的抗病毒分子转移的理解,并可能为控制病毒感染的治疗策略提供新的依据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号