Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational Analysis of Kinase Inhibitors Identifies Promiscuity Cliffs across the Human Kinome
ACS Omega ( IF 4.1 ) Pub Date : 2018-12-13 00:00:00 , DOI: 10.1021/acsomega.8b02998 Filip Miljković 1 , Jürgen Bajorath 1
ACS Omega ( IF 4.1 ) Pub Date : 2018-12-13 00:00:00 , DOI: 10.1021/acsomega.8b02998 Filip Miljković 1 , Jürgen Bajorath 1
Affiliation
|
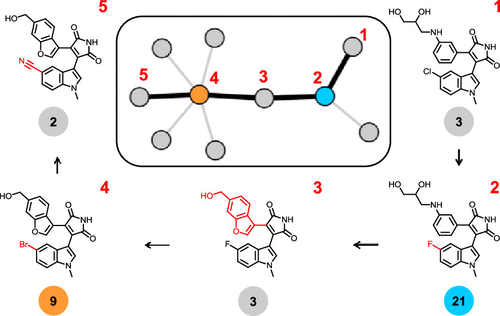
Kinase inhibitors are high-priority drug candidates for a variety of therapeutic applications. Accordingly, there has been a rapid growth in the number of kinase inhibitors and volumes of associated activity data. A paradigm for the use of kinase inhibitors in oncology is that these compounds have multitarget activities and elicit their therapeutic effects through polypharmacology. An analysis of kinase inhibitors and associated activity data from medicinal chemistry has so far only identified small subsets of highly promiscuous kinase inhibitors. In this study, we have collected inhibitors of human kinases and their activity data from seven public repositories, curated, and combined these data, yielding more than 112 000 inhibitors with well-defined activity measurements from which qualitative target annotations were derived. An analysis of these unprecedentedly large data sets revealed that nearly 40% of human kinase inhibitors have multikinase activities but that only 4% are known to be active against five or more kinases. However, structurally analogous inhibitors often displayed significant differences in the number of kinase annotations, leading to the formation of nearly 16 000 “promiscuity cliffs”. Moreover, 2236 promiscuity cliffs (14.03%) were formed by kinase inhibitors at different stages of clinical development. Overall, these cliffs suggested many target hypotheses for kinase inhibitors, taking data incompleteness into consideration, as well as hypotheses for structural modifications leading to kinase selectivity. Furthermore, from network representations, pathways comprising sequences of promiscuity cliffs were extracted that revealed unexpected structure–promiscuity relationships. To enable follow-up investigations, all promiscuity cliffs formed by human kinase inhibitors will be made freely available.
中文翻译:

激酶抑制剂的计算分析确定了整个人类基因组中的滥交悬崖。
激酶抑制剂是用于多种治疗应用的高优先级候选药物。因此,激酶抑制剂的数量和相关活性数据的数量迅速增长。在肿瘤学中使用激酶抑制剂的范例是这些化合物具有多靶标活性,并通过多药理学引起其治疗作用。迄今为止,从药物化学分析激酶抑制剂和相关活性数据只能鉴定出高度混杂的激酶抑制剂的一小部分。在这项研究中,我们从七个公共存储库中收集了人类激酶抑制剂及其活性数据,进行了整理,并将这些数据结合在一起,产生了超过112,000种具有明确活性测量结果的抑制剂,并由此得出了定性目标注释。对这些空前的大数据集的分析表明,近40%的人类激酶抑制剂具有多激酶活性,但已知只有4%的人对5种或更多种激酶具有活性。但是,结构类似的抑制剂通常在激酶注释的数量上显示出显着差异,导致形成近1.6万个“滥交悬崖”。此外,在临床发展的不同阶段,激酶抑制剂形成了2236个滥交悬崖(14.03%)。总体而言,这些悬崖暗示了激酶抑制剂的许多目标假设,同时考虑了数据的不完整性,以及导致激酶选择性的结构修饰假设。此外,根据网络表示,提取了包含滥交悬崖序列的通道,揭示了意想不到的结构-滥交关系。为了进行后续调查,将免费提供由人激酶抑制剂形成的所有滥交悬崖。
更新日期:2018-12-13
中文翻译:

激酶抑制剂的计算分析确定了整个人类基因组中的滥交悬崖。
激酶抑制剂是用于多种治疗应用的高优先级候选药物。因此,激酶抑制剂的数量和相关活性数据的数量迅速增长。在肿瘤学中使用激酶抑制剂的范例是这些化合物具有多靶标活性,并通过多药理学引起其治疗作用。迄今为止,从药物化学分析激酶抑制剂和相关活性数据只能鉴定出高度混杂的激酶抑制剂的一小部分。在这项研究中,我们从七个公共存储库中收集了人类激酶抑制剂及其活性数据,进行了整理,并将这些数据结合在一起,产生了超过112,000种具有明确活性测量结果的抑制剂,并由此得出了定性目标注释。对这些空前的大数据集的分析表明,近40%的人类激酶抑制剂具有多激酶活性,但已知只有4%的人对5种或更多种激酶具有活性。但是,结构类似的抑制剂通常在激酶注释的数量上显示出显着差异,导致形成近1.6万个“滥交悬崖”。此外,在临床发展的不同阶段,激酶抑制剂形成了2236个滥交悬崖(14.03%)。总体而言,这些悬崖暗示了激酶抑制剂的许多目标假设,同时考虑了数据的不完整性,以及导致激酶选择性的结构修饰假设。此外,根据网络表示,提取了包含滥交悬崖序列的通道,揭示了意想不到的结构-滥交关系。为了进行后续调查,将免费提供由人激酶抑制剂形成的所有滥交悬崖。



























 京公网安备 11010802027423号
京公网安备 11010802027423号