当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electron Correlations Engineer Catalytic Activity of Pyrochlore Iridates for Acidic Water Oxidation
Advanced Materials ( IF 29.4 ) Pub Date : 2018-12-14 , DOI: 10.1002/adma.201805104 Chunyan Shang 1 , Cong Cao 1 , Dayou Yu 1 , Yu Yan 1 , Yitao Lin 1 , Hongliang Li 1 , Tingting Zheng 1 , Xupeng Yan 1 , Wenchao Yu 1 , Shiming Zhou 1 , Jie Zeng 1
Advanced Materials ( IF 29.4 ) Pub Date : 2018-12-14 , DOI: 10.1002/adma.201805104 Chunyan Shang 1 , Cong Cao 1 , Dayou Yu 1 , Yu Yan 1 , Yitao Lin 1 , Hongliang Li 1 , Tingting Zheng 1 , Xupeng Yan 1 , Wenchao Yu 1 , Shiming Zhou 1 , Jie Zeng 1
Affiliation
|
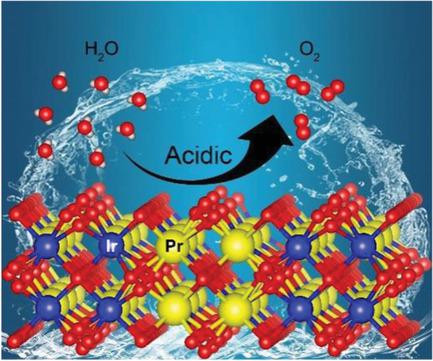
The development of highly efficient oxygen‐evolving catalysts compatible with powerful proton‐exchange‐membrane‐based electrolyzers in acid environments is of prime importance for sustainable hydrogen production. In this field, understanding the role of electronic structure of catalysts on catalytic activity is essential but still lacking. Herein, a family of pyrochlore oxides R2Ir2O7 (R = rare earth ions) is reported as acidic oxygen‐evolving catalysts with superior‐specific activities. More importantly, it is found that the intrinsic activity of this material significantly increases with the R ionic radius. Electronic structure studies reveal that the increased R ionic radius weakens electron correlations in these iridate oxides. This weakening induces an insulator–metal transition and an enhancement of IrO bond covalency, both of which promote oxygen evolution kinetics. This work demonstrates the importance of engineering the electron correlations to rationalize the catalytic activity toward water oxidation in strongly correlated transition‐metal oxides.
中文翻译:

电子相关工程师邻氯苯甲酸铱对酸性水氧化的催化活性
在酸性环境中开发与强大的基于质子交换膜的电解器兼容的高效析氧催化剂对于可持续制氢至关重要。在该领域中,了解催化剂的电子结构对催化活性的作用是必不可少的,但仍然缺乏。此处,一族的烧绿石氧化物R 2 Ir 2 O 7(R =稀土离子)据报道是具有优异比活性的酸性析氧催化剂。更重要的是,发现该材料的固有活性随R离子半径而显着增加。电子结构研究表明,增加的R离子半径削弱了这些铱酸盐氧化物中的电子相关性。这种减弱会引起绝缘体-金属的转变以及IrO键共价的增强,这两者都促进了氧气的释放动力学。这项工作证明了设计电子相关性以合理化强相关过渡金属氧化物中对水氧化的催化活性的重要性。
更新日期:2018-12-14
中文翻译:

电子相关工程师邻氯苯甲酸铱对酸性水氧化的催化活性
在酸性环境中开发与强大的基于质子交换膜的电解器兼容的高效析氧催化剂对于可持续制氢至关重要。在该领域中,了解催化剂的电子结构对催化活性的作用是必不可少的,但仍然缺乏。此处,一族的烧绿石氧化物R 2 Ir 2 O 7(R =稀土离子)据报道是具有优异比活性的酸性析氧催化剂。更重要的是,发现该材料的固有活性随R离子半径而显着增加。电子结构研究表明,增加的R离子半径削弱了这些铱酸盐氧化物中的电子相关性。这种减弱会引起绝缘体-金属的转变以及IrO键共价的增强,这两者都促进了氧气的释放动力学。这项工作证明了设计电子相关性以合理化强相关过渡金属氧化物中对水氧化的催化活性的重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号