当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Critical Assessment of the Interaction between DNA and Choline Amino Acid Ionic Liquids: Evidences of Multimodal Binding and Stability Enhancement
ACS Central Science ( IF 18.2 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1021/acscentsci.8b00601 Dipak Kumar Sahoo 1, 2 , Subhrakant Jena 1, 2 , Juhi Dutta 1, 2 , Suman Chakrabarty 3 , Himansu S. Biswal 1, 2
ACS Central Science ( IF 18.2 ) Pub Date : 2018-12-04 00:00:00 , DOI: 10.1021/acscentsci.8b00601 Dipak Kumar Sahoo 1, 2 , Subhrakant Jena 1, 2 , Juhi Dutta 1, 2 , Suman Chakrabarty 3 , Himansu S. Biswal 1, 2
Affiliation
|
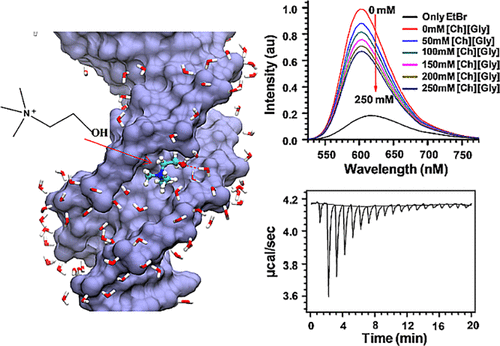
Long-term storage and stability of DNA is of paramount importance in biomedical applications. Ever since the emergence of ionic liquids (ILs) as alternate green solvents to aqueous and organic solvents, their exploration for the extraction and application of DNA need conscientious understanding of the binding characteristics and molecular interactions between IL and DNA. Choline amino acid ILs (CAAILs) in this regard seem to be promising due to their non-cytotoxic, completely biobased and environment-friendly nature. To unravel the key factors for the strength and binding mechanism of CAAILs with DNA, various spectroscopic techniques, molecular docking, and molecular dynamics simulations were employed in this work. UV–Vis spectra indicate multimodal binding of CAAILs with DNA, whereas dye displacement studies through fluorescence emission confirm the intrusion of IL molecules into the minor groove of DNA. Circular dichorism spectra show that DNA retains its native B-conformation in CAAILs. Both isothermal titration calorimetry and molecular docking studies provide an estimate of the binding affinity of DNA with CAAILs ≈ 4 kcal/mol. The heterogeneity in binding modes of CAAIL-DNA system with evolution of time was established by molecular dynamics simulations. Choline cation while approaching DNA first binds at surface through electrostatic interactions, whereas a stronger binding at minor groove occurs via van der Waals and hydrophobic interactions irrespective of anions considered in this study. We hope this result can encourage and guide the researchers in designing new bio-ILs for biomolecular studies in future.
中文翻译:

DNA和胆碱氨基酸离子液体之间的相互作用的关键评估:多峰结合和稳定性增强的证据。
DNA的长期存储和稳定性在生物医学应用中至关重要。自从离子液体(ILs)作为水性和有机溶剂的替代绿色溶剂出现以来,其对DNA的提取和应用的探索就需要认真理解IL和DNA之间的结合特性和分子相互作用。在这方面,胆碱氨基酸ILs(CAAILs)由于其无细胞毒性,完全基于生物和环境友好的性质而似乎很有希望。为了揭示CAAIL与DNA的强度和结合机理的关键因素,在这项工作中采用了各种光谱技术,分子对接和分子动力学模拟。UV-Vis光谱表明CAAIL与DNA的多峰结合,而通过荧光发射进行的染料置换研究证实了IL分子侵入DNA的小沟。圆二色光谱显示DNA在CAAIL中保留了其天然B构象。等温滴定量热法和分子对接研究都提供了DNA与CAAIL≈4 kcal / mol的结合亲和力的估计值。通过分子动力学模拟,建立了CAAIL-DNA系统结合方式随时间的异质性。胆碱阳离子在接近DNA时首先通过静电相互作用在表面结合,而在小沟上通过范德华力和疏水相互作用发生更强的结合,而与本研究中考虑的阴离子无关。我们希望这一结果能够鼓励和指导研究人员在将来为生物分子研究设计新的生物白介素。
更新日期:2018-12-04
中文翻译:

DNA和胆碱氨基酸离子液体之间的相互作用的关键评估:多峰结合和稳定性增强的证据。
DNA的长期存储和稳定性在生物医学应用中至关重要。自从离子液体(ILs)作为水性和有机溶剂的替代绿色溶剂出现以来,其对DNA的提取和应用的探索就需要认真理解IL和DNA之间的结合特性和分子相互作用。在这方面,胆碱氨基酸ILs(CAAILs)由于其无细胞毒性,完全基于生物和环境友好的性质而似乎很有希望。为了揭示CAAIL与DNA的强度和结合机理的关键因素,在这项工作中采用了各种光谱技术,分子对接和分子动力学模拟。UV-Vis光谱表明CAAIL与DNA的多峰结合,而通过荧光发射进行的染料置换研究证实了IL分子侵入DNA的小沟。圆二色光谱显示DNA在CAAIL中保留了其天然B构象。等温滴定量热法和分子对接研究都提供了DNA与CAAIL≈4 kcal / mol的结合亲和力的估计值。通过分子动力学模拟,建立了CAAIL-DNA系统结合方式随时间的异质性。胆碱阳离子在接近DNA时首先通过静电相互作用在表面结合,而在小沟上通过范德华力和疏水相互作用发生更强的结合,而与本研究中考虑的阴离子无关。我们希望这一结果能够鼓励和指导研究人员在将来为生物分子研究设计新的生物白介素。



























 京公网安备 11010802027423号
京公网安备 11010802027423号